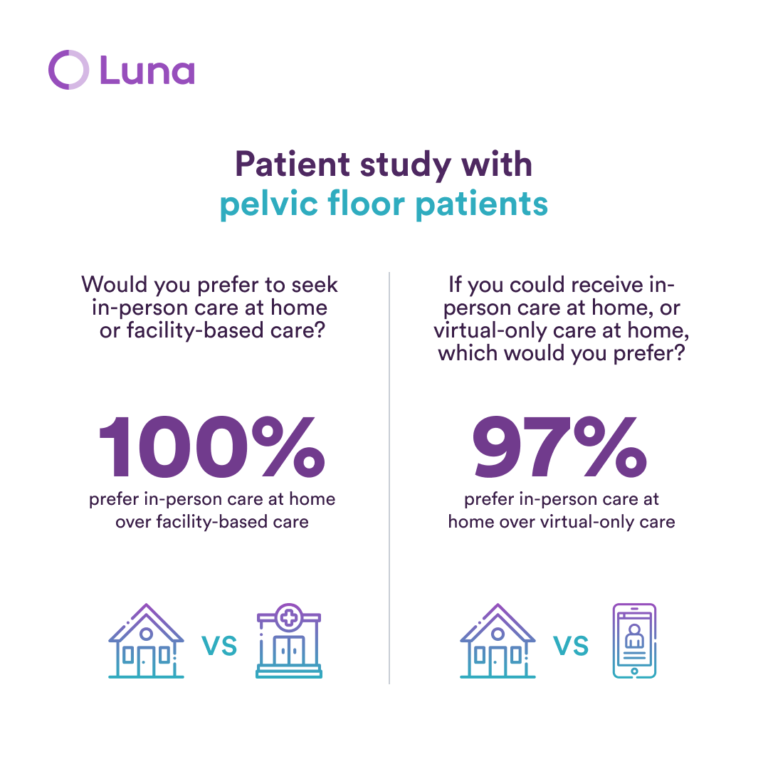
Luna, the leading provider of in-home physical therapy, has unveiled a new patient study, which casts doubt on virtual-only care for pelvic floor disorders.
In the study, 97% of patients with pelvic floor disorders preferred to receive in-person physical therapy at home, with only 3% favoring app-based care, and the majority of patients agreed that they “would not even seriously consider a virtual-only care option.”
The study revealed that 71% of patients receiving Luna’s in-home physical therapy for pelvic floor disorders showed statistically significant functional improvement with only 7 in-person visits needed on average.
Hinge Health recently launched a program for female pelvic floor disorders soon after its competitor SWORD Health announced its own pelvic health program. Luna provides effective pelvic floor care for all genders, in the familiarity, security, and privacy of their homes.
“The study demonstrates that in-person physical therapy, as opposed to virtual-only, is the go-to treatment for pelvic floor disorders. This means that companies, including Hinge Health and SWORD Health, are missing a huge opportunity for patient care,” said Palak Shah, Luna co-founder and head of clinical operations.
“Luna’s in-home physical therapy, accessible nationwide, provides effective pelvic floor care from the comfort and safety of their homes, while avoiding the hassles of going to a clinic, at no extra cost as treatment is covered by insurance,” added Shah.
To request in-home physical therapy care for pelvic floor disorders, patients can call or visit getluna.com, and no referral is needed to start treatment. Once requested, the physical therapist will arrive at the patient’s home equipped to treat the patient.
Luna is the nation’s fastest growing provider of on-demand, in-home physical therapy, with more than 4,000 therapists in 2022, treating a wide range of conditions. It operates in 42 U.S. markets and 24 states, and partners with some of the most innovative health systems nationally, including Emory Health, SCL Health, UCLA Health, and Scripps Health.
33% of women will experience a pelvic floor disorder in her lifetime, while 16% of men have been identified with a pelvic floor disorder, and is a growing concern as the world’s population ages. Pelvic floor disorders occur with weakened pelvic muscles or tears in the connective tissue, which may impact everyday physiological functions, such as controlling bowel and bladder continence and sexual function.