Penumbra, Inc. (NYSE: PEN), a global healthcare company focused on innovative therapies, announced that its Indigo Aspiration System with Lightning 7 and Lightning 12 have secured CE Mark (Conformité Européenne) and are now commercially available in Europe.
Both technologies are part of Penumbra’s Indigo Aspiration System – now with Intelligent Aspiration for mechanical thrombectomy – and are designed for single session arterial and venous thrombus removal, including the treatment of pulmonary embolisms.
“Technology advancements such as Lightning 7 and Lightning 12 are critical to improving patient outcomes and expanding use of mechanical thrombectomy to a broader range of patients,” said Dr. Andrew Wigham, interventional radiology consultant at John Radcliffe Hospital, Oxford, UK. “Lightning enables us to remove blood clots in the body quickly and efficiently, often in a single session, while also minimizing blood loss and potentially reducing the need for thrombolytics and prolonged ICU stays.”
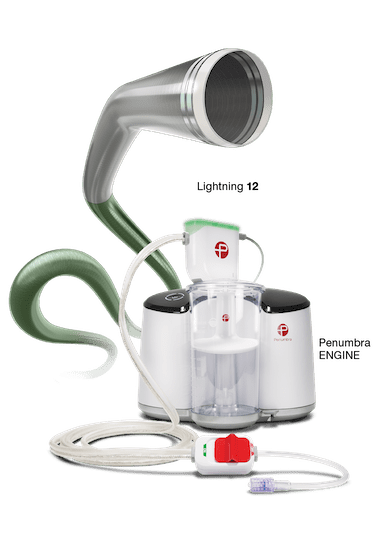
Powered by the Penumbra ENGINE®, Lightning 7 and Lightning 12 combine the new Indigo System CAT™7 and CAT12 Aspiration Catheters with Lightning Intelligent Aspiration, a unique computer-aided clot detection technology that can differentiate between clot and blood, designed to reduce blood loss and the need for clot-dissolving drugs, which may lower the risk of bleeding complications. The Lightning technology also provides an alternative to other surgical options. CAT7 is a high power, low profile catheter that features laser-cut hypotube technology and circumferential sweep designed for dependable delivery and maximal clot extraction. CAT12 is a large-lumen aspiration catheter that also incorporates novel laser-cut hypotube-based catheter engineering to provide advanced deliverability and torqueability within the body.
“The launch of our Lightning portfolio in Europe will provide a much-needed option for physicians to address debilitating blood clots in the body effectively and efficiently,” said James Benenati, MD, FSIR, chief medical officer at Penumbra, Inc. “We’ve seen our latest advancements in mechanical thrombectomy reduce blood loss while increasing clot removal efficiency in the US. These innovations may help improve patient care compared to conventional therapy. We are now able to help more patients with this technology by expanding access to physicians in Europe.”
“Blood clots in the body can be difficult to access and are potentially life-threatening. Until now, treatment options have been limited,” said Joan Kristensen, vice president and head of the Europe, Middle East and Africa region for Penumbra, Inc. “The introduction of our Lightning portfolio in Europe will expand access of our most advanced clot removal technology for the body, which combines intelligent aspiration and innovative catheter engineering to remove blood clots in a single session. With Lightning, mechanical aspiration technology continues to advance to meet the needs of patients, which is our core purpose at Penumbra.”
About Indigo System with Lightning Intelligent Aspiration
The Indigo System with Lightning is an intelligent aspiration system powered by Penumbra ENGINE. Lightning Aspiration Tubing has dual pressure sensors for real-time blood flow monitoring. This unique mechanism of action helps optimize thrombus removal procedures by differentiating between thrombus and blood. The system is designed to aspirate continuously when in thrombus and intermittently in patent flow. Throughout the case, Lightning provides procedural feedback via audiovisual cues. Lightning’s thrombus removal algorithm is designed to initiate automatic valve clicking when it senses patent flow. With automatic valve control, Lightning is designed to help reduce blood loss and allow the physician to focus on optimizing thrombus removal procedures.
The Indigo System with Lightning Intelligent Aspiration and Separators is indicated for the removal of fresh, soft emboli and thrombi from the peripheral arterial and venous systems, and for treatment of pulmonary embolism.