January 27, 2021
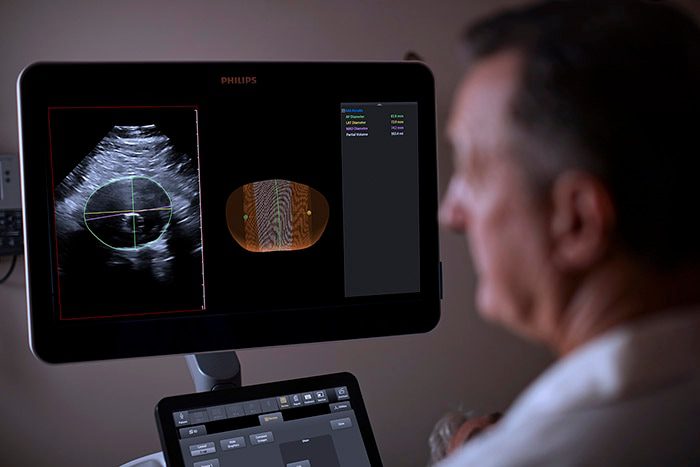
Philips Abdominal Aortic Aneurysm (AAA) Model is based on 3D ultrasound, Philips AAA Model delivers clinicians accurate diagnostic information without exposing patients to high doses of radiation and nephrotoxic contrast agents.
An abdominal aortic aneurysm (AAA) is an aneurysm that forms in the lower part of the aorta. Typically, AAAs are identified incidentally during abdominal imaging exams but, in some cases, remain undetected until rupture. A ruptured AAA has an 80% mortality rate [1], emphasizing the importance of routine surveillance. Philips AAA Model integrates innovative software and leading Philips 3D ultrasound technologies into a single solution to help increase diagnostic confidence and an improved patient experience. The software automatically segments and quantifies the size of the aneurysm sac for surveillance of known native (untreated), and post-EVAR (treated) AAAs.
The current standard of care for AAAs includes 2D ultrasound and computed tomography angiography (CTA). Each of these modalities has its drawbacks, including inter-operator variability with 2D ultrasound and patient exposure to high levels of radiation and nephrotoxic contrast agents with CTA.
A recent clinical study showed that 3D ultrasound examination for native AAA surveillance has excellent inter-operator reproducibility, superior to that of 2D ultrasound, supporting the broader use of 3D ultrasound in standard AAA surveillance programs [2]. 3D ultrasound has been shown to estimate the diameter and volume of an AAA with acceptable reproducibility and an improved agreement (over 2D ultrasound) with CT [3]. Furthermore, 3D ultrasound has also been proven to correlate significantly better to 3D CT than 2D ultrasound for assessing the maximum diameter of the residual sac post-EVAR, with clinically acceptable reproducibility [4].
Note: Philips will debut the AAA Model at the LINC (Leipzig Interventional Course) Summit, Europe’s premiere interventional course for vascular specialists, taking place virtually, Jan. 25-29, 2021.
The Philips Abdominal Aortic Aneurysm (AAA) Model is CE marked for sale in Europe and is FDA cleared for sale in the U.S.
[1] Age-specific incidence, risk factors, and outcome of acute abdominal aortic aneurysms in a defined population. Howard DP, Banerjee A, Fairhead JF, et al. Br J Surg. 2015;102(8):907–915. doi:10.1002/bjs.9838
[2] Clinical validation of three-dimensional ultrasound for abdominal aortic aneurysm. Ghulam, Qasam M. et al., Journal of Vascular Surgery, Volume 71, Issue 1
[3] Three-dimensional ultrasound evaluation of small asymptomatic abdominal aortic aneurysms. Bredahl, K. et al. European Journal of Vascular and Endovascular Surgery, Volume 49, Issue 3, 289 – 296
[4] Three-dimensional ultrasound improves the accuracy of diameter measurement of the residual sac in EVAR patients. K. Bredahl, M. Taudorf, A. Long, L. Lönn, L. Rouet, R. Ardon, H. Sillesen, J.P. Eiberg. European Journal of Vascular and Endovascular Surgery, Volume 46, Issue 5, 2013