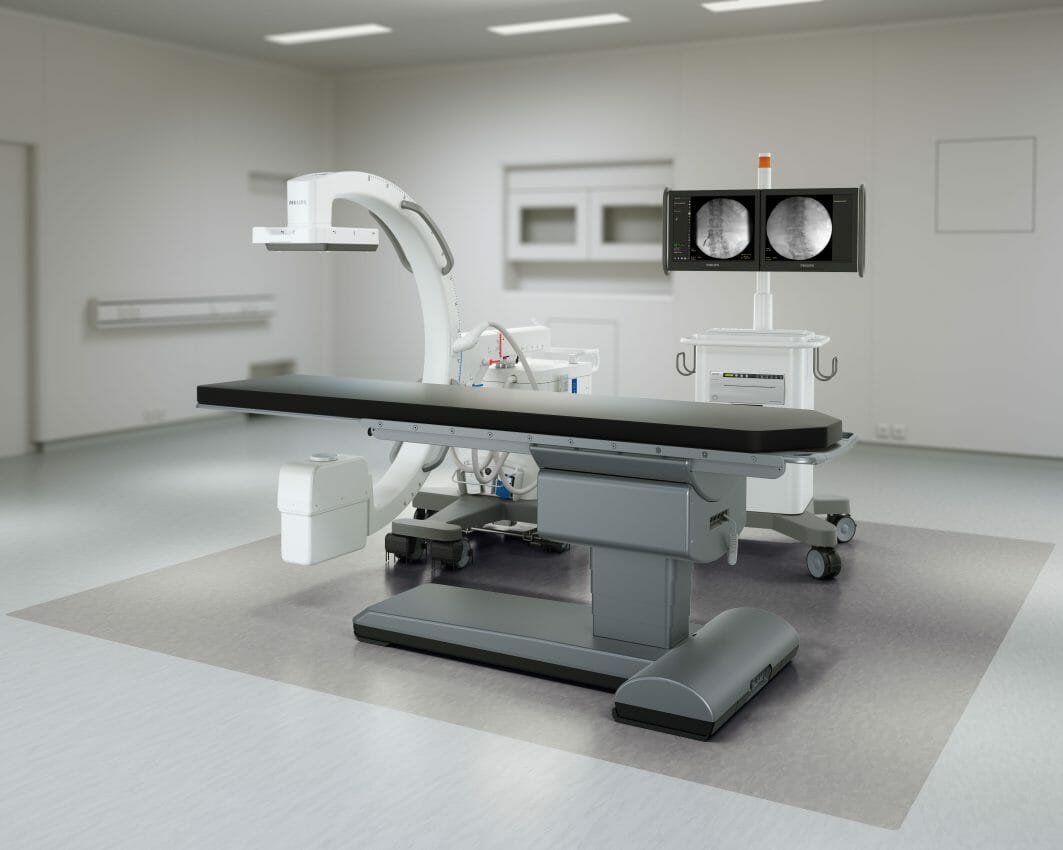
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Philips Image Guided Therapy Mobile C-arm System 1000 – Zenition 10, a new addition to the company’s Zenition mobile C-arm series. Based on Philips’ state-of-the-art flat panel detector technology, Zenition 10 helps to expand patient access to routine surgical care and minimally invasive procedures. Now commercially available [1], the cost-effective, high-utilization, high-image-quality mobile C-arm enables surgeons to treat more patients at a lower cost while helping improve patient outcomes.
Mark Stoffels, General Manager, Image Guided Therapy Systems at Philips:
“Philips Zenition mobile C-arms have long been recognized for their high-quality imaging and efficiency-enhancing performance. With the introduction of Zenition 10, we are making those benefits available on a C-arm that meets the wide-ranging needs of routine open and minimally invasive surgery, allowing more patients in more parts of the world to receive the high-quality care.”
“Philips Zenition mobile C-arms have long been recognized for their high-quality imaging and efficiency-enhancing performance. With the introduction of Zenition 10, we are making those benefits available on a C-arm that meets the wide-ranging needs of routine open and minimally invasive surgery, allowing more patients in more parts of the world to receive the high-quality care,” said Mark Stoffels, General Manager, Image Guided Therapy Systems at Philips.
Mitigating staff shortages, increasing access to high-quality care
To help alleviate operating room staff shortages and rising healthcare costs, Philips Zenition 10 provides a cost-effective imaging solution for routine surgery, delivers the speed and efficiency needed to deal with high patient throughput, while being flexible enough to meet the needs of orthopedics, trauma, and other areas of surgery to maximize utilization. It comes with exceptional C-arm maneuverability, application-specific protocols, and personalized user profiles. At the same time, it delivers the excellent image quality needed to improve patient outcomes. User-friendly and intuitive to operate, the Zenition 10 supports a fast learning curve and reduces operating staff training time. Next to this, the new system offers a dedicated low-dose pediatric mode.
Consistent image quality is facilitated by application-specific protocols, customizable presets, and unique user profiles that automatically adjust Zenition 10 settings to suit an individual user’s preferences whenever they log in. An uninterruptible power supply allows the unit to be moved from one operating room to another without the need to reboot the entire system. Its excellent C-arm geometry provides the maneuverability needed for operating room staff to access target anatomies. Philips BodySmart facilitates dose efficiency by automatically adapting the measuring field to the area of interest.
For images of patients with metal implants, Philips MetalSmart software automatically enhances image quality and dose efficiency. A dedicated pediatric mode automatically reduces dose rates for young patients. The system’s digital subtraction angiography capability further enhances the range of surgical procedures facilitated by the Zenition 10, allowing clear imaging of vasculatures.
The Zenition mobile C-arm platform brings together innovations in image capture, image processing, ease-of-use, and versatility pioneered on Philips’ highly successful and Windows® based Zenition platform. Other C-arm systems in Philips’ Zenition series are the Zenition 50 and Zenition 70.
[1] Zenition 10 mobile C-arm systems are available for sale in a limited number of countries. Please check with a local representative for availability in your market.