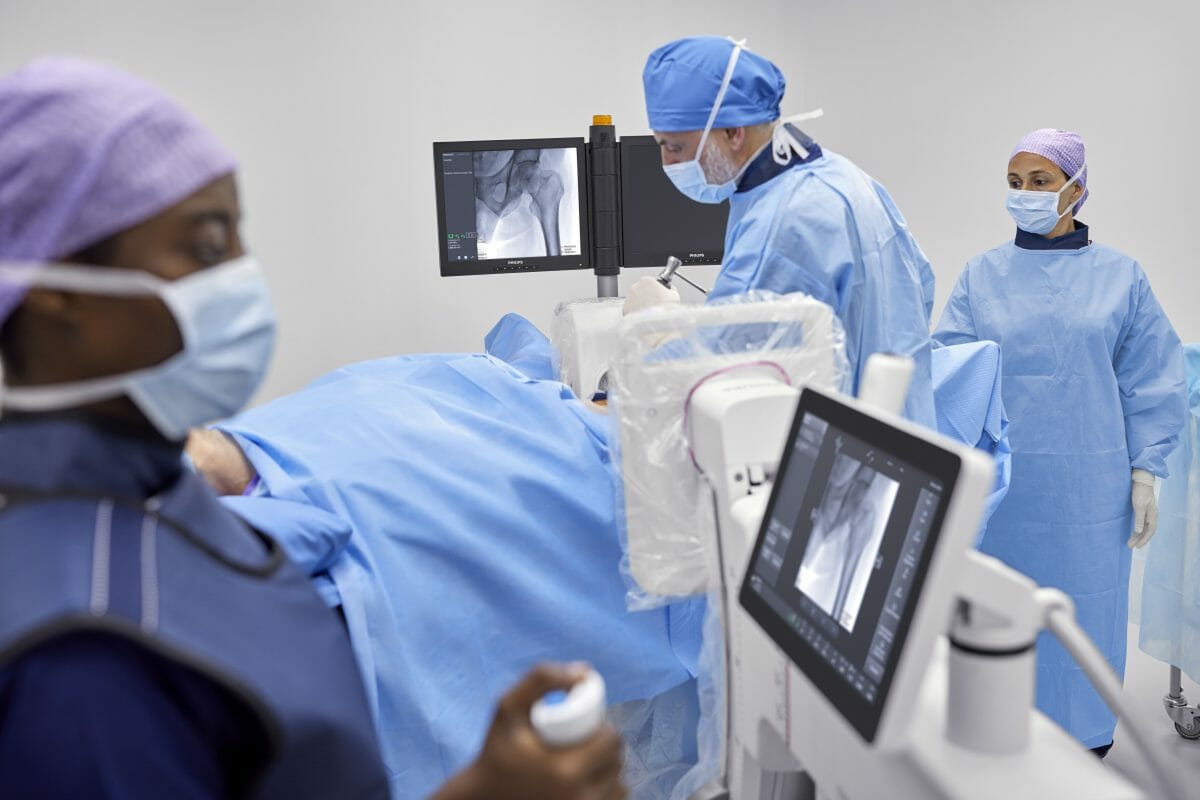
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced the launch of Philips Image Guided Therapy Mobile C-arm System 3000 – Zenition 30 [1], the latest addition to the company’s highly versatile Zenition mobile C-arm series. Mobile C-arms are X-ray systems that are brought into the operating room (OR) to provide live image guidance during a wide range of clinical procedures which include orthopedics, trauma, spine, pain management and other surgical procedures.
The Zenition 30 gives surgeons a greater level of control, empowering them to work more autonomously and independently. By reducing dependency on support staff, Zenition 30 helps alleviate the staff shortages faced by many hospitals, while its workflow-enhancing features and excellent image quality allow surgeons to deliver high-quality care to more patients. The launch of the Zenition 30 follows the recent launch of the Philips Zenition 10 C-arm [2].
“Based on our Zenition platform’s proven ease of use and workflow efficiency, the new Zenition 30 offers a unique combination of personalized control and image clarity to enhance the speed and accuracy of decision-making for a range of clinical procedures at a price point that meets today’s economic and business goals,” said Mark Stoffels, General Manager, Philips Image Guided Therapy Systems.
High-quality imaging
Zenition 30 offers versatility for a range of clinical procedures which include orthopedics, trauma, spine, pain management and other surgical procedures. Featuring Philips’ latest-generation flat detector technology, advanced imaging algorithms, and personalized user profiles, the new system delivers superior image quality, dose efficiency, and workflow customization. From the moment a surgeon logs on, the Zenition 30 automatically adjusts to their preferred settings and way of working, contributing to fewer manual adjustments and more first-time-right imaging. In addition to rapid set-up and protocol selection, the Zenition 30 also offers surgeons much greater control over C-arm positioning during surgical procedures.
In independent usability studies involving clinicians around the world who were offered hands-on experience of the Zenition 30 in simulated environments, 95% said they believed its enhanced surgeon control would allow them to work independently [3], while 84% believed that Zenition 30’s personalized image quality profiles meant fewer images might be needed during a procedure because the first image already incorporates their preferred settings [4].
As part of its commitment to doing business sustainably and helping its customers make responsible choices, Philips has designed the Zenition 30 to have a 25% increased product lifetime [5] and also has a refurbished program available, making it ‘circular-ready’ for repair, refurbishment, and recycling [5].
Other C-arm systems in Philips’ Zenition series are the Zenition 50, Zenition 70, and the recently launched Zenition 10 [2].
[1] Zenition 30 mobile C-arm systems are available for sale in a limited number of countries. Please check with a local representative for availability in your market. Pending 510 (k) not available for sale in the USA.
[2] Zenition 10 mobile C-arm system is not available in the USA.
[3] Results were obtained during a claims substantiation study performed in February and September 2022 by Use-Lab GmbH, an independent company. Response is based on 37 clinicians around the world, who answered a questionnaire subsequent to a usability study with additional hands-on time with the system.
[4] Results were obtained during a claims substantiation study performed in February and September 2022 by Use-Lab GmbH, an independent company. Response is based on 50 clinicians around the world, who answered a questionnaire subsequent to a usability study with additional hands-on time with the system.
[5] Compared to its predecessor, BV Endura 2.3.