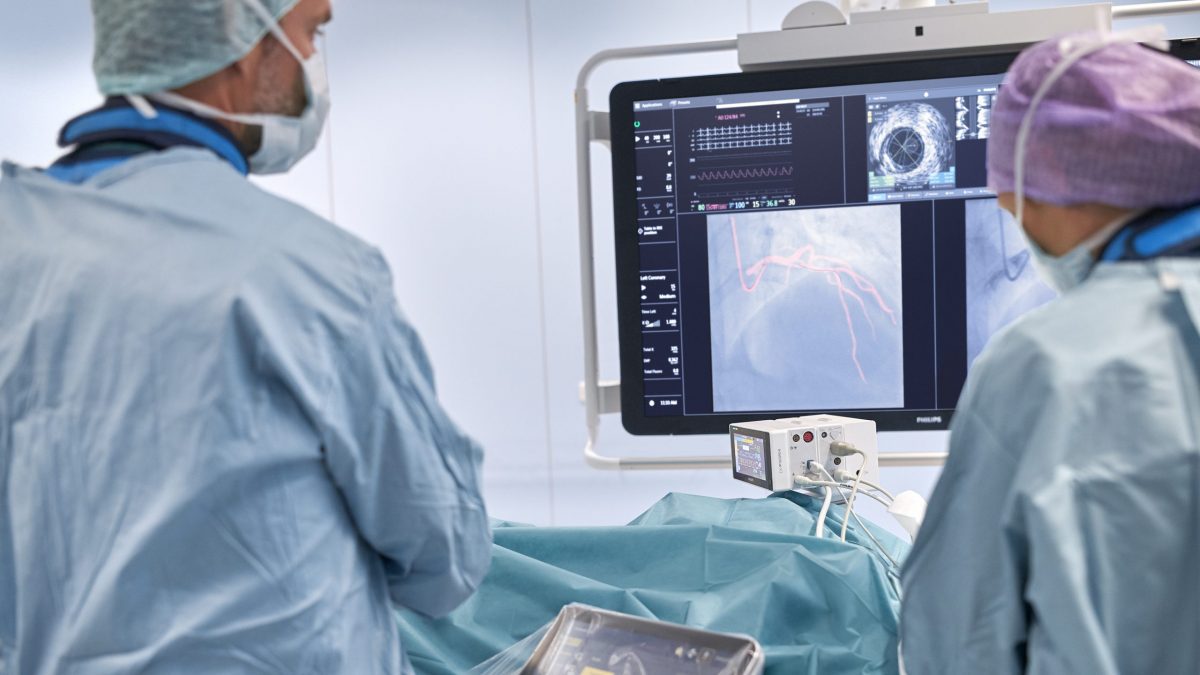
Philips today announced the integration of its Interventional Hemodynamic System and market-leading portable Patient Monitor IntelliVue X3, providing advanced hemodynamic (blood flow) measurements at the tableside in the cath lab and continuous monitoring of key vital signs throughout the patient journey.
The integration provides the opportunity for monitoring during image-guided procedures on the Philips Image Guided Therapy System – Azurion, improving workflow with comprehensive patient records that support timely clinical decision-making during interventional cardiology procedures and beyond. The solution will be introduced at ACC.21, the American College of Cardiology’s 70th Annual Scientific Session and Expo, taking place virtually on May 15-17.
Health systems strive to deliver consistent quality patient care while optimizing the interventional room to treat more patients and accelerate care of the critically ill. Uninterrupted patient monitoring, as patients are moved from holding areas to the interventional room and then on to recovery areas, can help to improve clinical decision making and the timely detection of potential adverse events at every stage of the patient journey. Incorporating a 12-lead diagnostic ECG, the monitor removes the need to re-cable the patient or reconnect monitoring, and can help lead to reduced procedural preparation time, enhanced staff efficiency, and ultimately an enhanced patient experience.
“Philips Interventional Hemodynamic System with Patient Monitor IntelliVue X3 is a unique combination made possible by our strengths in patient monitoring and image-guided therapy,” said Ronald Tabaksblat, General Manager Image-Guided Therapy Systems at Philips. “Bringing advanced hemodynamic measurements to the tableside as an integrated part of our Azurion image-guided therapy system, it helps allow clinicians to assess the condition of the patient in real-time during an intervention, without leaving the sterile field. It also streamlines workflows, providing continuous monitoring of the patient during every step of their care path, enhancing patient safety and confidence.”
The IntelliVue X3 patient monitor can be connected to the patient from anywhere in the hospital, after which it stays with them throughout the interventional procedure, providing continuous monitoring and registration of their vital signs in the IntelliVue IT solution.
After connecting the IntelliVue X3 to its docking station at the tableside of the Azurion system, clinical staff can monitor all of the patient’s vital signs including pulse oximetry end-tidal CO2, perform hemodynamic analyses and 12-lead ECG acquisitions, and relay results and waveforms from the lab’s control room to the Azurion tableside display. From the tableside the interventionalists can assess the data using the Azurion tableside Touch Screen Module, allowing them to remain focused on their patients. The system brings the latest physiological techniques to the interventional lab, including iFR (instant wave-Free Ratio) measurements, a hyperemia-free technique unique to Philips that provides valuable functional information regarding the severity of lesions in the coronary arteries.
Philips Interventional Hemodynamic System with Patient Monitor IntelliVue X3 integrates with Azurion, the company’s next-generation image-guided therapy system that allows clinicians to easily and confidently perform procedures with a unique user experience, helping to optimize lab performance and provide superior care. At ACC.21 Philips will showcase its solutions for the diagnosis and treatment of Structural Heart Disease, which aim to remove the barriers associated with complex procedures by helping to deliver clinical confidence where it is needed most – at the point of treatment. Philips’ cardiac care solutions help strengthen clinical confidence, build efficiency throughout the care pathway, and enhance care experiences.