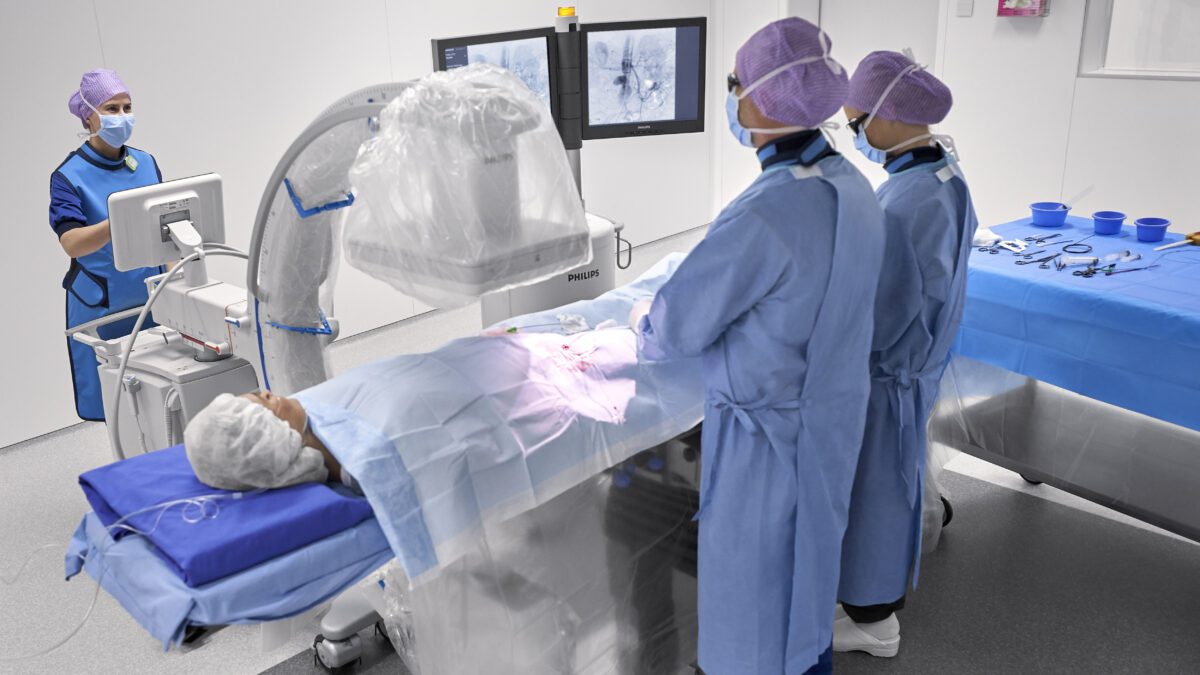
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today announced physicians will now have access to advanced new 3D image guidance capabilities through its Image-Guided Therapy Mobile C-arm System – Zenition, delivering enhanced clinical accuracy and efficiency, and aiming to improve the outcomes for patients undergoing endovascular treatment. The company has signed a strategic partnership agreement with Cydar, a UK-based provider of cloud-based procedure maps software to plan and guide surgery in real time.
As patient numbers rise and procedures become more complex and time-consuming, patient-specific real-time procedure planning and guidance, optimization of equipment utilization, and usability have become ever more important. To help overcome these challenges, Philips Image-Guided Therapy Mobile C-arm System – Zenition – brings together innovations in image capture and processing, ease-of-use, and versatility, many of which were pioneered on Philips’ highly successful image guided therapy platform Azurion. Like Azurion, the Zenition mobile C-arm system allows hospitals to maximize operating room performance, enhance their clinical capabilities, and provide staff with a seamless user experience. The integration of Cydar EV Maps software into the Zenition platform now adds extended procedure planning and real-time 3D guidance capabilities.
Cloud-based AI and computer vision
Cydar EV Maps assists in the planning, real-time guidance, and post procedure review of endovascular surgery. It brings cloud-based AI and computer vision to mobile surgery, enabling reductions in radiation exposure, fluoroscopy time, and procedure time together with improved ease of use [1,2]. It enables surgeons to create a detailed patient-specific 3D map of the target vasculature to help plan surgery, and then uses these maps to augment intraoperative live image-guidance, updating the maps in real time to account for deformations during surgery, such as guidewires and instruments deforming the patient’s blood vessels. Cydar EV also facilitates post procedure outcome analysis. The combined result of this integration of procedure planning, guidance and review is that surgeons can work more accurately and efficiently – enabling an approximate 50% reduction in radiation exposure [1], a significant reduction in fluoroscopy time and a reduction of procedure times by more than 20% [2], while helping achieve better outcomes for patients.
“With Philips’ integrated portfolio, using validated AI and cloud technologies, we can facilitate collaborative care to optimize surgical pathways,” said Karim Boussebaa, General Manager Image Guided Therapy Systems at Philips. “Philips has a strong global network of mobile surgery systems and recognizes that Cydar’s EV Maps solution can play a key role in further improving our integrated offering for endovascular procedures.”
“By partnering with Philips to bring our Cydar EV Maps solution to Philips’ user-friendly Zenition platform of mobile C-arms, we are a step closer to achieving our mission to ensure every image-guided minimally-invasive surgical procedure goes exactly as planned,” said Paul Mussenden, CEO of Cydar. “The benefits of this combined solution, with integrated and enhanced procedure planning and visualization, include shorter and more predictable procedure times, reduced X-ray exposure to patients and staff, and fewer injections of iodine dye – the leading cause of in-hospital kidney failure.”
Cydar EV Maps is currently in use across the EU, UK and US. It is certified Software-as-a-Medical Device with EU CE mark and U.S. FDA 510(k) clearances.
References
[1] Southerland K, Nag U, Turner M, Gilmore B, McCann R, Long C, Cox M, Shortell C. IF09. Image-Based Three-Dimensional Fusion Computed Tomography Decreases Radiation Exposure, Fluoroscopy Time, and Procedure Time During Endovascular Aortic Aneurysm Repair. J Vasc Surg. 2018;67: e61-e61
[2] Shortell C., Veithsymposium New York, 2017