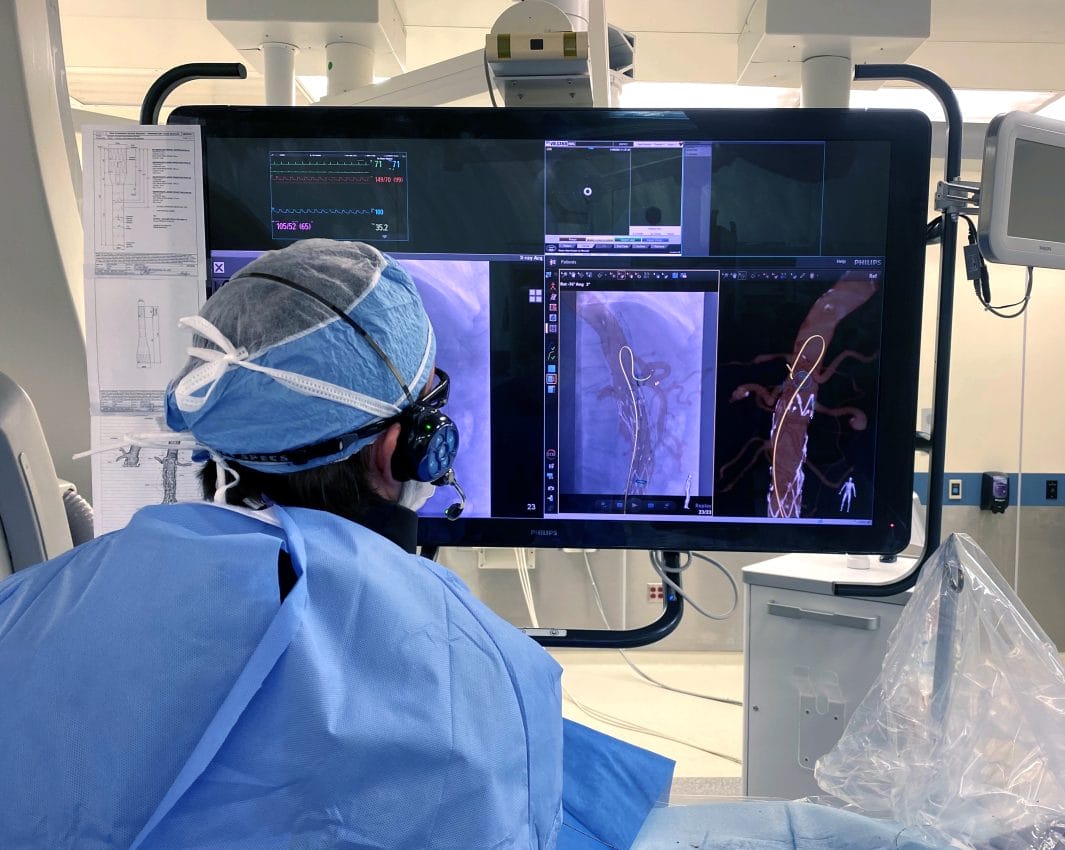
More than 100 years since the X-ray was discovered, Philips introduces a breakthrough innovation to the world of healthcare imaging. LumiGuide, powered by Fiber Optic RealShape (FORS) technology, enables doctors to navigate through blood vessels using light, instead of X- ray. “[It’s] one of the most exciting changes that we’ve seen with imaging certainly throughout my career,” said Andres Schanzer, Vascular Surgeon, at UMass Memorial Medical Center, Worcester, MA, USA. In late 2023, LumiGuide was used for the first time to treat patients in Maastricht University Medical Center in the Netherlands, closely followed by the University of Alabama at Birmingham in the USA. Developed in close collaboration with clinical partners, LumiGuide is made available, in first instance, to major Aortic Centers of Excellence that perform complex aortic repairs in the USA and Europe.
“[It’s] one of the most exciting changes that we’ve seen with imaging certainly throughout my career,” – Andres Schanzer, Vascular Surgeon, at UMass Memorial Medical Center, Worcester, MA, USA.
LumiGuide’s radiation-free technology brings potentially game-changing benefits for complex aortic procedures. In vascular surgery, physicians often perform endovascular surgery using devices like guidewires and catheters, via, for example, the femoral artery. This is also referred to as an endovascular procedure. For many decades clinicians had to only rely on X- ray to guide their devices through blood vessels. But x-ray can do both harm as well as good. In addition to its potentially harmful radiation, x-ray can only produce 2D black and white images. With physicians increasingly tackling far more complex endovascular procedures – such as aortic aneurysm repair – cases take more time, resulting in a higher radiation dose for patients and clinicians. |
LumiGuide uses light reflected along an optical fiber inside a guidewire to generate 3D, high-resolution, color images of devices, including off-the-shelf catheters, inside a patient’s body in real time, from any angle and in multiple views. It means that physicians know which direction their device is facing and can see where they need to go. This navigation can be done all without X-ray.
“If we can see more, we can proceed more quickly and more confidently,” said Dr. Atul Gupta, Chief Medical Officer for Image Guided Therapy and Precision Diagnosis at Philips and practicing interventional radiologist. “In effect, LumiGuide is a 3D human GPS system powered by light.”
Moving at the speed of light: Fast procedures, minimal radiation
Having worked on this technology for the past years, Philips has been able to already yield promising study results around the world. Following a limited release to nine aortic centers, more than 900 patients have undergone procedures – with one site, conducting a historic cohort comparison, showing a 37% reduction in complex aortic procedure time, and a 56% reduction in radiation exposure (DAP) compared to X-ray.[1]
Integrating AI into this second-generation innovation
LumiGuide, which works exclusively with compatible Philips interventional systems like Azurion, is the second-generation solution to use FORS. Building on insights, data and clinical feedback from the first-generation devices used at the nine centers, LumiGuide includes new time-saving features. Instead of doctors having to manually register their devices with their image-guided therapy platform, LumiGuide uses AI-based recognition to register the guidewire quickly and efficiently, helping to increase accuracy while speeding up procedure time even more. All the doctor needs to do is confirm the wire’s registration.
Prof. Geert Willem Schurink, vascular surgeon at Maastricht University Medical Center, who performed the first surgical procedure with LumiGuide, said: “This AI-based semi-automatic registration is very quick and accurate, even in the presence of stent grafts. Especially, if there is a need to re-register the device being guided in the patient’s body during the procedure, it is extremely helpful.”
As a next step, LumiGuide will enable Philips and clinical partners to gather more clinical data on the performance at existing sites in preparation of making the solution available globally.
One step closer to radiation-free minimally-invasive surgery
With LumiGuide now available to a growing number of aortic centers in the USA and Europe, Philips is paving the way for new radiation-free procedures. With plans to enable more devices that can be guided by LumiGuide, beyond aortic procedures, Philips is working towards bringing healthcare closer to entirely radiation-free surgery – which ultimately means easier, faster, safer care for patients and the clinicians who care for them.
[1] 37% procedure time reduction, 56% radiation reduction (DAP) during complex aortic repair. Clinical research study, Initial single-center experience using Fiber Optic RealShape guidance in complex endovascular aortic repair, Journal of Vascular Surgery, E. Finnesgard, A. Schanzer; Nov 2022