Luna, the leading provider of in-home physical therapy, today announced that Providence, a not-for-profit health system serving the Western U.S., will expand access to outpatient physical therapy service through the Luna technology platform.
The Luna technology platform will match eligible Providence patients to therapists based on factors, including specialty, geography, and schedules. Luna’s broad network of local physical therapists will provide consistent, high-quality care, of the type administered in a traditional outpatient clinic, but from the comfort of a patient’s home.
“Providence is an innovative health system that is continuously finding ways to meet patients where they are,” said Prasanna Mohanty, chief operating officer for the clinical network at Providence. “We recognize that there is a growing number of physical therapy outpatients needing or wanting to remain at home, and Luna’s in-home model will enable our teams to accommodate patient needs and ensure quality care that is safe and convenient.”
The platform will allow patients and therapists to communicate with each other and discuss care needs between visits. The same therapist will treat the patient for the entirety of the treatment plan, which will ensure consistent and quality care. Between visits, patients will use the Luna platform to communicate with their physical therapists, set appointment times, and perform therapist-prescribed and monitored exercises.
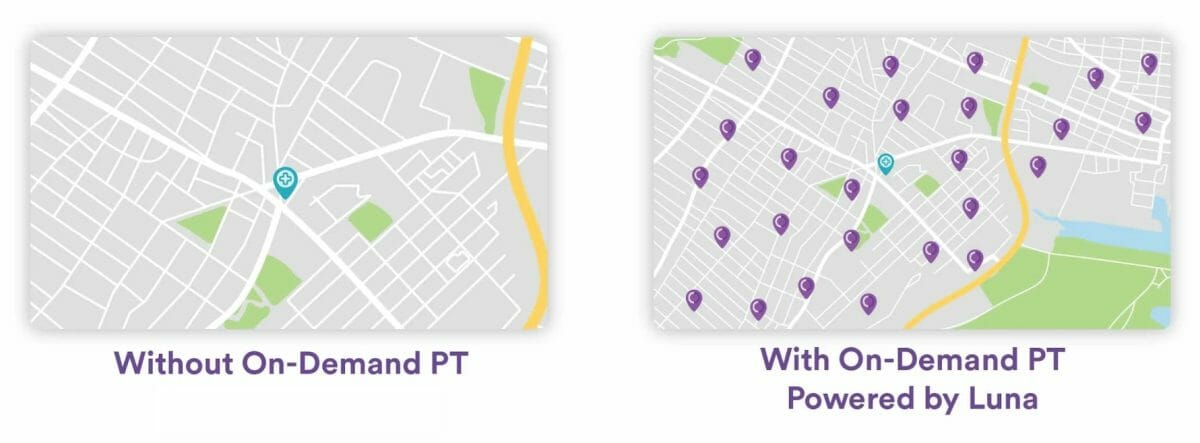
“Patients demand greater flexibility in outpatient services, including the ability to choose when and where services are performed,” said Palak Shah, co-founder and head of clinical operations at Luna. “The addition of Luna’s in-home physical therapy provides relief for health systems operating at capacity and needing more access. This will allow more patients within the Providence health system to complete their treatment plan at a convenient place and time — and ensure quality and continuity of care.”
Luna will accept all insurance providers contracted with Providence, with the exception of worker’s compensation and disability.