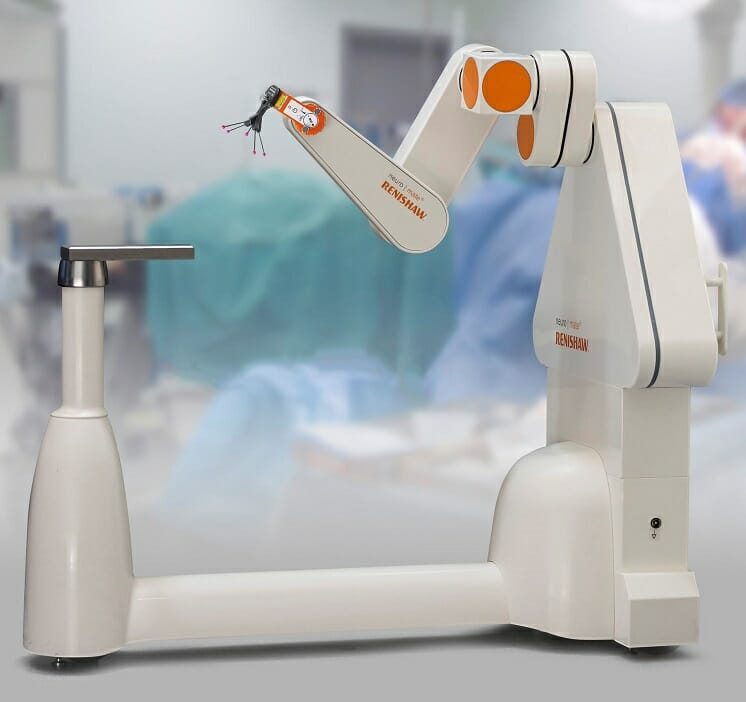
Renishaw, a global engineering company pioneering robotic neurosurgical solutions, announces that the company’s neuromate® stereotactic robot, incorporating neuroinspire™ surgical planning software, has received EU Medical Device Regulation (MDR) certification from its Notified Body, the British Standards Institution (BSI).
As one of the first companies to achieve MDR approval, Renishaw reaffirms its commitment to the market, its customers and, most importantly, the patients who will continue to benefit from the use of its products.
In a move that became one of the most significant shifts in European medical device regulations in over two decades, the MDR 2017/745 replaced the European Directives on Medical Devices (AIMDD 90/385/EEC and MDD 93/42/EEC) in May 2021. The goal was to create a more modernised, robust and long-term legislative framework for medical devices, as well as software that is used by those devices, with strict inspection by Notified Bodies to assure the highest levels of safety and health.
The introduction of the MDR 2017/745 means more stringent requirements, particularly in the area of clinical and post-market review data, are in place. As a result, many medical device companies within the EU have had to make significant time and financial investments to improve their current processes to capture and analyse this data.
“Achieving MDR certification is a significant achievement for Renishaw and is the result of months of hard work, dedication and due diligence within our team”, said Nina Sainte-Marie, Operations Manager at Renishaw. “Without this and the early adoption of the new regulatory requirements in Europe, certification would not have been possible. I am especially proud to be part of a team that values quality so highly and is committed to establishing compliance within the new EU regulations.”
Paul Skinner, General Manager at Renishaw, commented: “The replacement of the MDD with the MDR has improved the standards for medical device regulation within Europe and we are delighted to be one of the first neurosurgical solutions companies to receive certification. Not only does it reaffirm our commitment to compliance with the evolving regulatory requirements for medical devices but is testament to our progress in meeting the standards required for approval of the neuromate robot and neuroinspire planning software. Thanks to the Renishaw team we’re able to continue supply within Europe and reach our ultimate goal of ensuring patients have access to life-changing neurosurgical devices.”