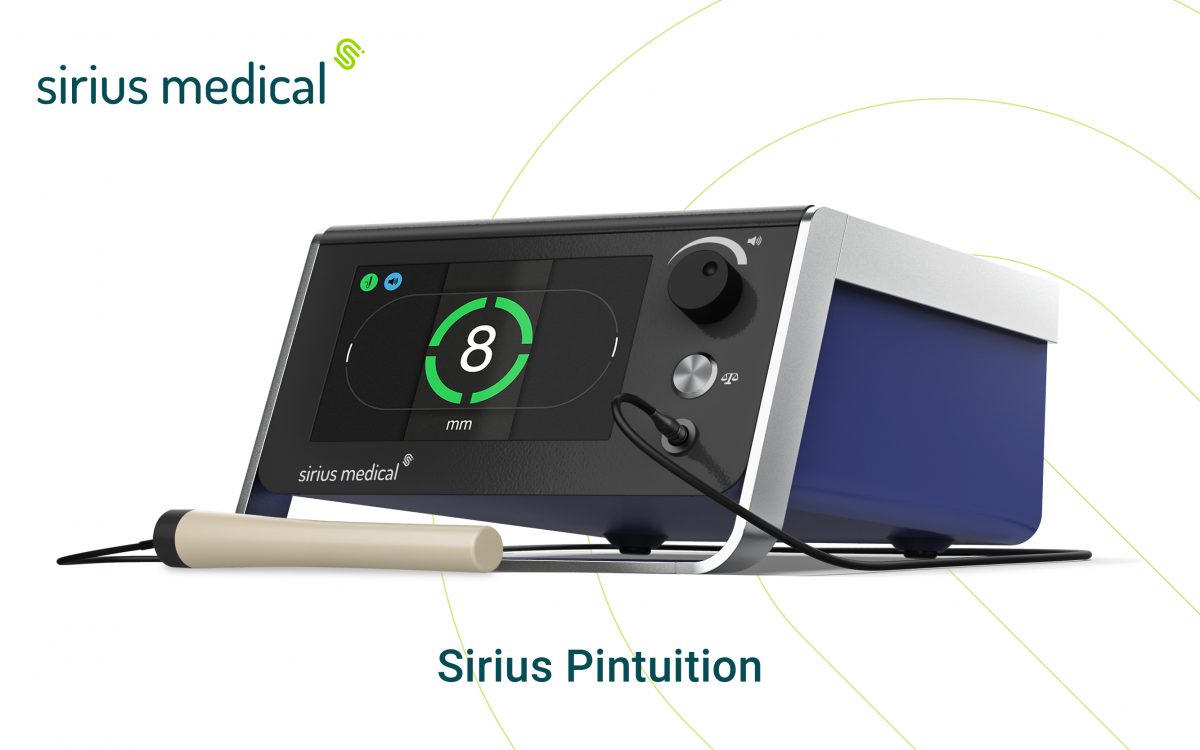
Sirius Medical, a leader in tumor localization technology, introduces the latest version of the Sirius Pintuition® system powered by a state-of-the-art navigation software, GPSDetect™.
The advanced software provides real-time, directional guidance using audio and visual feedback for unmatched precision to locate tumors, enabling surgeons to navigate to the tumor more easily and precisely. The Pintuition platform with GPSDetect will be revealed at the upcoming 40th European Society of Surgical Oncology (ESSO) Conference in Lisbon, Portugal.
“Our latest GPSDetect software uses our unique multi-sensor technology to provide surgeons unmatched guidance and accuracy,” says Bram Schemers, CEO Sirius Medical. “Pintuition is highly intuitive and our TargetLOC™ feature provides additional visual feedback to the operator when the probe is perfectly aligned above the seed.”
The Sirius Pintuition system is a state-of-the-art navigation technology for the treatment of non-palpable breast cancer and other soft tissue tumor types. The new system, enhanced by GPSDetect and TargetLOC, delivers a better alternative to surgeons by offering an easy-to-use and reusable detection system that needs only a single calibration per procedure, is highly robust, and cannot be de-activated or lose its signal. Surgeons, radiologists, and staff can easily learn the technology, and it replaces current localization procedures without additional adoption investments.
“The GPSDetect software provides many advantages,” says breast surgical oncologist Irma den Hoed of ETZ hospital in Tilburg, the Netherlands. “Having both visual and audio directional feedback provides perfect navigation towards the tumor and allows me to perform an optimal oncoplastic lumpectomy while significantly improving and simplifying the surgical procedure.”
“We are pleased to use Sirius Pintuition with GPSDetect for targeted soft tissue surgery,” says breast surgical oncologist Isabel Rubio, Breast unit Clinica Universidad de Navarra, Madrid Spain. “The updated software greatly improves localization in breast cancer surgery and is easier and more accurate to use. We look forward to partnering with Sirius Medical to train more surgical oncologists on localization techniques for guided breast cancer surgery.”
“Sirius Pintuition provides precise and directional navigation towards small, non-palpable lesions,” says surgical oncologist Riccardo Giovanazzi, Head Breast Cancer Surgery and Director Breast Unit San Gerardo Hospital, Monza, Italy. “It is very competitive with other technologies supporting surgeons during breast cancer conserving surgery.”
“Sirius Medical has demonstrated they are an innovative company by rapidly responding to surgeons’ needs and producing GPSDetect, which has the promise of providing both distance and navigational guidance for seed localization within the breast and axilla. The iBRA-NET team looks forward to evaluating this technology,” says Mr. Edward St John, Innovation Group Chair for iBRA-NET and Consultant Oncoplastic Breast Surgeon at Portsmouth Hospitals University NHS Trust, UK.
Recently Sirius Medical achieved the milestone of performing over 1,000 procedures for the treatment of non-palpable breast cancers. The Sirius Pintuition system is CE marked and is currently commercially available in Western Europe. The company also expanded operations to the U.S. earlier this year upon receiving FDA clearance.
“We are proud to offer a technology designed by physicians for physicians and are excited about co-creating the future direction of cancer surgery,” says Jan Willem Beijer, Chief Commercial Officer at Sirius Medical. “Sirius Pintuition is helping more physicians precisely and efficiently remove early-stage tumors and provide optimal care for breast cancer patients.”