Smart Noise Cancellation (SNC) technology by Carestream has received FDA 510(k) Clearance for its dose reduction capability.
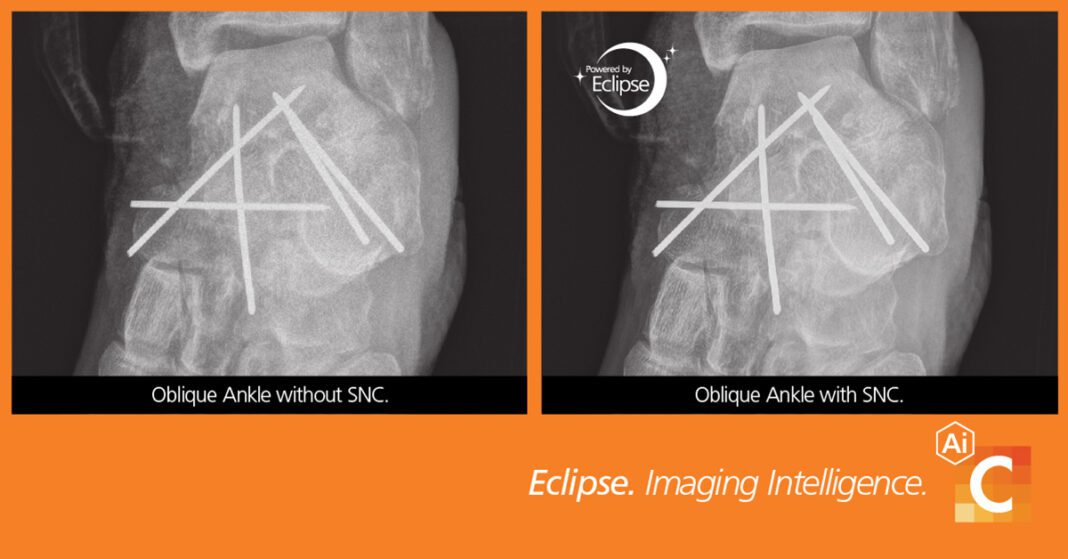
The artificial intelligence (AI)-based feature allows a user to reduce dose without loss of image quality.* SNC provides improved diagnostic quality, preservation of fine detail and better contrast-to-noise ratio for images acquired at clinically nominal exposures.
“Carestream is continuously focused on reducing radiation dose without sacrificing image quality,” said Ron Muscosky, Worldwide Product Line Manager at Carestream. “The results of this reader study are another proof point of the quality of our high-performance image capture systems. This improved capability to optimize radiation dose will be especially valuable in neonatal and pediatric diagnostic imaging, where imaging at the lowest possible dose is crucial for young patients.”
SNC addresses a long-standing challenge in medical imaging of separating noise from an image. Traditional noise reduction introduces blurring, which degrades image sharpness and might remove important anatomical information. Conversely, the more an image is sharpened, the more noise may be enhanced. Carestream’s SNC overcomes this gridlock by isolating noise to produce images that are significantly clearer than with standard processing.
“Our AI-powered Smart Noise Cancellation gives radiologists another important tool to adjust the amount of noise cancellation and exposure to meet their desired imaging quality to aid their diagnosis,” Mr. Muscosky added.
When combined with SmartGrid software, Smart Noise Cancellation technology promises benefits in gridless imaging where the removal of scatter typically leads to an increase in noise appearance.
Smart Noise Cancellation software is available as an option with Carestream’s ImageView Software powered by Eclipse—the intelligent image-processing engine behind the company’s innovative imaging software. SNC is currently available on Carestream’s DRX-Evolution and
DRX-Evolution Plus systems.
*These statements were verified using Carestream detectors in a reader study performed by board certified radiologists comparing pairwise images taken at nominal dose (CsI ISO 400 speed / GOS ISO 320 speed) and reduced dose (CsI ISO 800 speed / GOS ISO 500 speed) with SNC.