9Smith+Nephew (LSE: SN), (NYSE: SNN), the global medical technology company, today announces the first robotic-assisted surgery using its LEGION CONCELOC Cementless Total Knee System was performed by Dr. Cyna Khalily, an orthopaedic surgeon specializing in adult reconstructive surgery of the hip and knee in Louisville, KY.
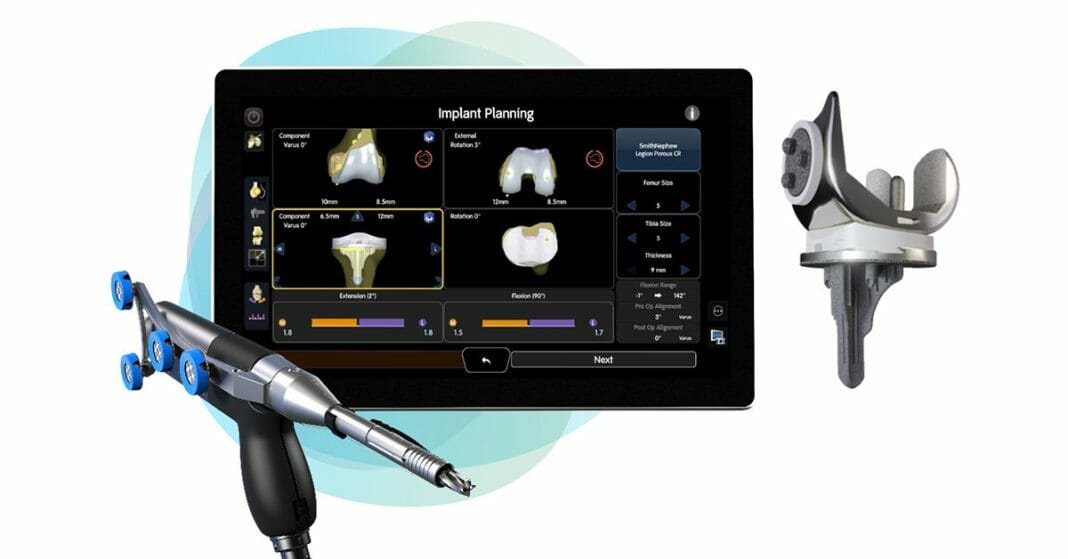
Smith+Nephew is the only medical device company to offer both total and partial cementless knee implants, and now, a robotic-assisted solution for cementless total knee arthroplasty (TKA) with the CORI◊ Surgical System. The LEGION CONCELOC Cementless Total Knee System addresses the critical elements of the cementless total knee through its unique design. The asymmetrical keel is designed to provide immediate rotational stability, while the patented 3D printed structure of CONCELOC Advanced Porous Titanium encourages biological ingrowth.
The CORI Surgical System is a second-generation platform that is small, portable, and capable of performing robotic-assisted knee and computer-guided hip surgery on a single platform. Unlike other systems, it eliminates time, costs, and radiation exposure1 associated with preoperative CT imaging.
“The accuracy2 of precision milling, combined with the tactile feel of the press-fit and initial stability of the CONCELOC porous structure during implantation, are going to give surgeons the confidence to perform cementless total knee procedures,” said Dr. Cyna Khalily.
Smith+Nephew continues to provide technological innovation with its robotics platform, coupled with advanced materials science and a history of successful clinical performance*3-6 with leading implants, like the LEGION CONCELOC Cementless Total Knee System.
“As an orthopaedics company focused on driving technology enabled procedures, it is important for us to continue delivering on our promises and provide customers innovative, comprehensive solutions, like our cementless knee portfolio which now includes a TKA solution enabled by CORI handheld robotics,” said Randy Kilburn, Executive Vice President & General Manager, Orthopaedic Reconstruction, Robotics and Digital for Smith+Nephew.
Smith+Nephew is uniquely positioned to accommodate a comprehensive suite of procedural needs for primary implant solutions using enabling technologies and handheld robotics.
References
- Ponzio DY, Lonner JH. Preoperative Mapping in Unicompartmental Knee Arthroplasty Using Computed Tomography Scans Is Associated with Radiation Exposure and Carries High Cost. J Arthroplasty. 2015;30(6):964-967
- Kaper BP, Villa A. Accuracy and Precision of a Handheld Robotic-guided Distal Femoral Osteotomy in Robotic-assisted Total Knee Arthroplasty. European Knee Society Arthroplasty Conference;2019; Valencia, Spain.
- Victor J, Ghijselings S, Tajdar F, et al. Total knee arthroplasty at 15-17 years: does implant design affect outcome? Int Orthop. 2014;38(2):235-241.
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) 2021. Hip, Knee & Shoulder Arthroplasty Annual Report.
- National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Hertfordshire, UK 2021. 18th Annual Report.
- McCalden RW, Hart GP, MacDonald SJ, Naudie DD, Howard JH, Bourne RB. Clinical Results and Survivorship of the GENESIS II Total Knee Arthroplasty at a Minimum of 15 Years. J Arthroplasty. 2017;32(7):2161-2166