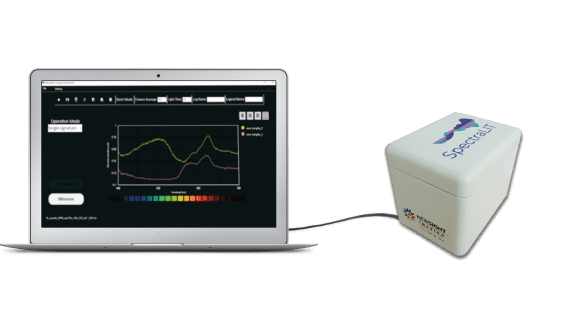
Newsight Imaging, the CMOS image sensor chip maker for 3D machine vision and spectral analysis applications announced the release of its SpectraLIT, a compact development kit suitable for testing Newsight technology and developing proof-of-concept devices for the healthcare and food & beverage industries or other liquids analysis.
SpectraLIT™ is a development kit based on Newsight’s spectrometer-on- chip. It allows for spectral profiling of substances in wavelengths between 400 to 700 nm in a matter of seconds. The next generation of SpectraLIT™️ will be able to support wavelengths between 400 to 1100 nm. The kit will allow diagnostic and analytical companies a fast market introduction of a portable, and low-cost diagnosis device. SpectraLIT is controlled by a Windows-based demo SW that displays, analyzes and stores the spectral profiles of the tested substance.
SpectraLIT uses standard cuvettes, which are easily inserted through a slide covered by a top lid, allowing full isolation of the tested substance from any external ambient light.
Using a special developed algorithm, SpectraLIT can compare the tested substance’s spectral profiles to profiles stored in a local database, allowing detection and identification of matching substances previously analyzed by SpectraLIT.
Newsight is now offering its potential partners to develop its POC based on SpectraLIT™. Upon achieving successful implementation, Newsight is offering its partner to license the technology, acquire the spectral chips or use a ready-made module to build their own game changing device.
On June 16th, 16:00 pm CEST, Newsight will conduct a free live webinar demonstrating SpectraLIT™ for potential future partners who will be interested in building an immediate, portable, and affordable detection device based on spectral profiling technology. To register here.