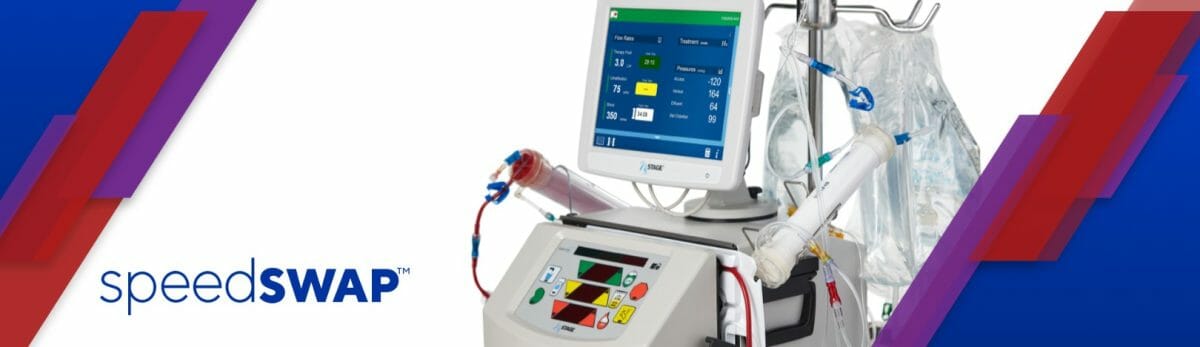
Fresenius Medical Care North America’s (FMCNA) Renal Therapies Group today announced the availability of Speedswap®, a new option for the NxStage® System One™ with NxView™ that enables the changing of a flow-compromised dialysis filter without replacing the entire cartridge. The new Speedswap system is designed to help reduce therapy downtime,1 ease nursing workloads,1 and lower treatment costs2 in acute care settings.
Clogging or clotting of the dialysis circuit is a common occurrence associated with continuous kidney replacement therapy (CKRT) in critical care settings, which can lead to therapy downtime.3 Speedswap allows a flow-compromised NxStage filter to be replaced without replacing the cartridge through the use of a pre-attached, yet detachable, filter.
“This introduction of Speedswap aims to both improve the quality of dialysis treatment in the critical care setting and make our technology even easier to use by the care team,” said Joe Turk, President of the Renal Therapies Group at Fresenius Medical Care North America. “This product is another example of how we are always working to introduce innovations that improve our NxStage System One and ensure it remains the first and best choice of all hospitals for continuous therapy.”
The innovative system brings to the market two products to be used with the NxStage System One with NxView — Cartridge Express with Speedswap and Speedswap Express Kit.
Some of the features of the Speedswap cartridge system include:
Shipping connectors designed to maintain the sterility and integrity of valves during storage and the shipping process.
Valves that enable the user to disconnect the pre-attached filter. The valves are designed to stop the flow of fluid when disconnected and allow fluid to flow when connected.
A line restrictor that is designed to allow for pressure adjustment within the venous and arterial lines, as well as to allow for connections to alternate sources of access.
Safe2 Rotator Connector that features a spin-lock designed to prevent accidental disconnect and improve handling for greater flexibility and durability.
Pressure Oscillating Diaphragm (POD) Technology that is created to eliminate the blood-air interface and may reduce the risk of filter clotting and related therapy interruptions.
Needleless valves designed to minimize the occurrence of air in the circuit.
“The simplified filter exchange should help make our life-sustaining therapy even easier to provide in an often busy critical care setting,” said Dr. Mike Anger, Chief Medical Officer for FMCNA’s Renal Therapies Group. “We also hope to improve patient outcomes by reducing the time necessary to change dialysis filters which should minimize interruption to continuous kidney replacement therapy.”
The NxStage System One with NxView is used by leading U.S. hospitals including the majority of the top renal care hospitals as ranked by US News & World Report.4 With a uniquely designed volumetric fluid balancing system, fluid accuracy is achieved without the need for scales. Simple, intuitive controls are designed to ensure ease of use and simplify user experience, while scale-based alarms are completely eliminated, reducing staff workload.
Effluent flows to an open drain line, eliminating interruptions in therapy due to emptying waste bags, and the system eliminates the need for cumbersome equipment and water treatment systems.
For more information about Speedswap, please visit here.