Tracheobronchomalacia: An Underrecognized Challenge
Tracheobronchomalacia (TBM) is characterized by the weakening of the cartilage and then collapse of the airway walls, leading to severe respiratory difficulties. Despite its debilitating effects, and its prevalence TBM often goes underdiagnosed due to overlapping symptoms with other respiratory disorders like Asthma and COPD. Patients experience a range of symptoms from seal-like coughing and frequent respiratory infections to life-threatening breathing struggles. Traditional treatments involve invasive surgical procedures that come with high risks and prolonged recovery times.
The Crucial Need for Advanced Treatment Options
TBM affects an estimated 8 million people in the US alone. Current treatment options for TBM are limited and often fail to provide long-term relief or improved quality of life for patients. The patented LM Scaffold System™ aims to address this critical need by offering a safer treatment option. This evolutionary device provides structural support to the weakened airway walls and is designed to help patients and provide improvement just days after the surgery.
The LM Scaffold System™: A Product Evolution
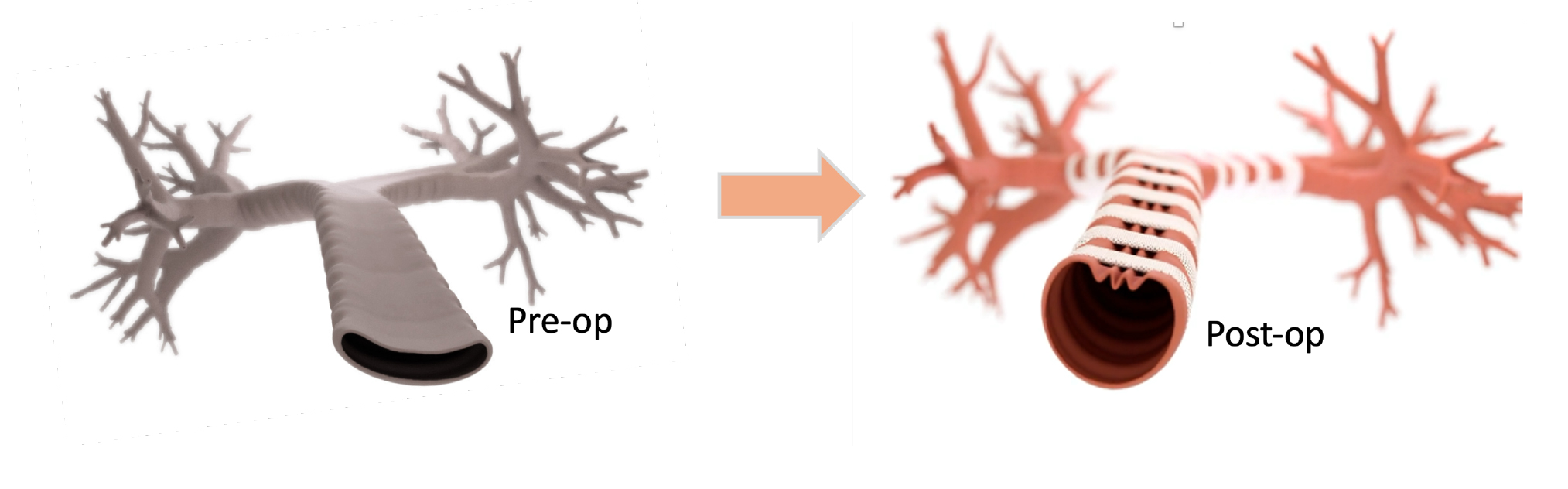
The LM Scaffold System™ represents an advancement in TBM treatment. This innovative device is designed to provide structural support to the trachea and bronchi, restoring their normal function much more efficiently than the current product used today for TBM repair via a tracheobronchoplasty. It can be implanted either through an open tracheobronchoplasty (TBP) or robotically. When combined with minimally invasive, robotically-assisted implantation process, The LM Scaffold System™ aims to create repeatable surgical intervention while minimizing patient recovery time. The second FDA pre-submission meeting is scheduled for early July. It is anticipated that the LM Scaffold System™ will be cleared next year.
Why the LM Scaffold System™ is Different
The LM Scaffold System™ is designed to impact patients with TBM in several ways:
- Minimally Invasive Approach: Traditional TBM treatments involve open surgery, which is highly invasive and comes with significant risks and recovery times. The LM Scaffold System™, on the other hand, can be implanted using a minimally invasive, robotically-assisted technique, which is designed to reduce surgical trauma, lower the risk of complications, and shorten recovery times.
- Repeatability and Standardization: The robotically-assisted Minimally Invasive Tracheal Repair (MITR™) technique employed with the LM Scaffold System™ is designed to standardize surgical repair. This standardization can help ensure that more surgeons, regardless of experience level, can achieve similar results. The LM Scaffold System™ helps “level the playing field,” and can enable less experienced surgeons to perform the surgery similarly to their more seasoned counterparts.
- Specific Design: Unlike standard mesh used for airway repairs, the LM Scaffold System™ features two interlocking pieces that connect like a zip tie. It is designed to enable a secure and stable reinforcement of the airway, and a clear view of the tracheal anatomy allowing robotic surgeons to help reinforce the structural integrity and function of the trachea and bronchi. It is estimated that an FDA-clearance of a product for the remediation of TBM in the form of the LM Scaffold System™ may reduce time in the operating room by approximately 50%.
A Dual Approach to TBM Treatment
The LM Scaffold System™ and the minimally invasive tracheal repair (MITR™) procedure are not separate solutions but rather help become complementary parts of a unified approach. Early in our research, we discovered that many patients with progressive symptoms were not responding to treatments because their airway, not their lungs, was the root cause of their issues. This realization led to a dual approach: the development of the LM Scaffold System™ and the refinement of the minimally invasive surgical repair technique (MITR™).
The Benefits of the LM Scaffold System™
The LM Scaffold System™ is designed to help:
- Improve Breathing: By stabilizing the airway, the LM Scaffold System™ is anticipated to help restore the natural anatomy and function of the trachea and bronchi. This can help patients experience improvements in their ability to inhale and exhale, reducing shortness of breath and improving overall respiratory function.
- Lessen Debilitating Symptoms: The design of the LM Scaffold System™ can allow patients to breathe easier and reduce symptoms like chronic coughing and recurrent infections. This improvement in respiratory function can be life-changing, allowing patients to engage in daily activities and social interactions without the debilitating symptoms of TBM.
- Reduce Recovery Times: The minimally invasive, robotically-assisted implantation process can enable patients to experience less surgical trauma, leading to faster recovery times and a quicker return to normal activities.
The Technology Behind the Innovation
The core of Lazzaro Medical’s innovation lies in its unique approach to the structural support of the trachea and bronchi. Utilizing advanced robotics and state-of-the-art imaging technology, the company has developed a minimally invasive procedure that can reduce the risks associated with traditional surgery. This new technique not only aims to be safer but much more efficient, reducing surgery time by as much as 50% and in doing so can create faster recovery times and better long-term outcomes for patients.
Collaboration + Grant = Future Prospects
The development of this innovative treatment is the result of collaboration between Lazzaro Medical and leading medical researchers and institutions. The company has recently been awarded a $2.4 million Small Business Innovation Research (SBIR) Direct to Phase II grant from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). The grant will further enhance these collaborative efforts, supporting ongoing research and development efforts, verification and validation, preclinical work, and regulatory activities.
The partnership Lazzaro Medical has with top-tier medical researchers and institutions, including Mayo Clinic and Northwell Health has been crucial in advancing this technology. The grant will enable the acceleration of research and move the medical world closer to providing a viable treatment option for TBM patients worldwide.
Impact on the Medical Community
The implications of Lazzaro Medical’s innovation extend beyond TBM. The development of a minimally invasive, robotically-assisted platform for airway treatment could pave the way for similar advancements in other areas of respiratory medicine. This breakthrough has the potential to set a new standard in the treatment of airway conditions, offering a safer, more effective alternative to traditional surgical methods.
Patient-Centric Care
At the heart of Lazzaro Medical’s mission is a commitment to patient-centric care. By focusing on minimally invasive techniques, the company aims to improve the patient experience, reducing pain, recovery times, and the overall impact of treatment on patients’ quality of life. This approach will not only benefit patients physically but can also contribute to better mental and emotional well-being by minimizing the stress and anxiety associated with invasive medical procedures and long recovery.
The Road Ahead
With strategic funding from institutions such as Northwell Health, Mayo Clinic and a recently awarded Direct to Phase II SBIR grant from the National Institutes of Health (NIH), Lazzaro Medical is poised to make strides in its mission to improve patient care for those suffering from Tracheobronchomalacia (TBM) by advancing the development of its robotically assisted, Minimally Invasive Tracheal Repair (MITR™) procedure, using its patented LM Scaffold System™. The goal is to bring this innovative treatment to TBM patients.
The support from the NIH is a crucial step in a critical journey. It enables Lazzaro Medical to continue its focus to bring innovative diagnostics and treatment to those who need it most and highlights the importance of eradicating this degenerative disease. The LM Scaffold System™ is the first step in a broader platform to enhance diagnosis and treatment of patients with TBM. Improved diagnostics and standardization of surgical repair can help facilitate adoption and fuel growth. The LM Diagnostic Scope™ which is being designed for use “in office” to shorten the time and effort to correctly and quickly diagnose TBM and the LM DuLET™, a more efficient patent-pending non-occlusive double lumen endotracheal tube that is designed to further simplify the MITR™ procedure and other surgeries.
LM Scaffold System™, LM DL Tube™ and LM Scope™ are not cleared or approved products by regulatory bodies in the US or internationally and are pending FDA submission.
Editor’s Comments: Richard Lazzaro, MD is a world-renowned thoracic surgeon with over 35 years of specialized expertise in minimally invasive and robotic surgery. With a deep commitment to advancing medical technology and improving patient outcomes, Dr. Lazzaro leads the development of innovative treatments for tracheobronchomalacia and other respiratory conditions. His visionary approach and dedication to patient-centric care have positioned Dr. Lazzaro at the forefront of medical innovation.
For more information about Lazzaro Medical and their innovative treatments, visit Lazzaro Medical’s website.
