February 3, 2021
Transit Scientific today announced the close of its Series A financing round. The round was led by a large multinational investor and joined by previous seed investors.
Transit Scientific achieved FDA clearances and multi-center, multi-user, safe-patient-use of its XO Score angioplasty scoring-cutting platform and separate XO Cross 0.014″ and 0.035″ microcatheter platform in late 2020.
“XO Score successfully dilated multiple lesions that were resistant to standard or high-pressure balloon angioplasty during our 1st patient uses in hemodialysis fistulas,” said Jeffrey Hoggard MD, of the Raleigh Access Center in Raleigh, North Carolina, USA. “It was particularly beneficial to be able to deploy the XO Score device with a standard PTA balloon in the juxta-anastomotic position and dilate the lesion at low and controlled inflation pressure.”
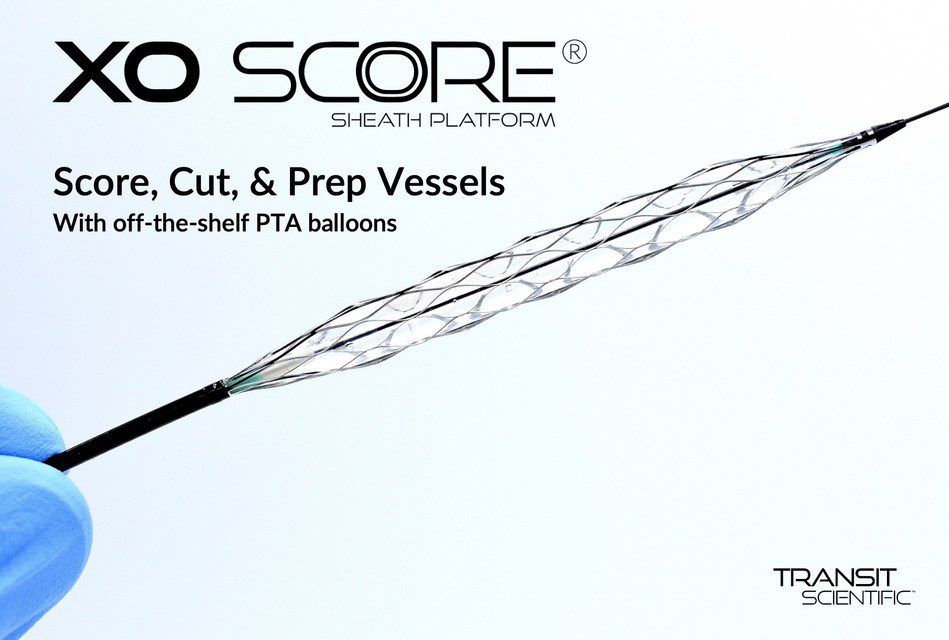
The XO Score is a novel sheath that converts regular angioplasty balloons into vessel prep scoring and cutting systems. The device is indicated for treatment of peripheral vascular stenosis including hemodialysis fistulas and grafts.
XO Score’s 22 individual scoring & cutting struts rotate 90 degrees to score & cut upon balloon inflation and then rotate 90 degrees back upon balloon deflation to facilitate balloon re-wrap. The technology also allows for infusion at the site of treatment.
“The XO Cross 014, 018, and 035 microcatheter platform adds new torque, pushability and catheter control,” said Richard Saxon, MD, FSIR of the Tri-City Medical Center in Oceanside, CA, USA “We have used an .014 XO Cross to redirect the guidewire intraluminally while crossing a tibial artery CTO. This would have been very difficult to achieve with other available technology.”
The XO Cross is a non-tapered exoskeleton based micro-catheter technology designed to improve torque response, trackability, and control.
“We are grateful to have smart value-added investors,” said Greg Method, president and chief executive officer of Transit Scientific. “Our team puts patients first and achieved a great deal in 2020 including FDA clearances and, most importantly, safe-patient-uses in a variety of challenging procedures with cardiologists, interventional radiologists, nephrologists, and vascular surgeons at different locations.”
Transit Scientific is a private company that designs, develops, and commercializes medical devices including the FDA-cleared XO ScoreÒ scoring sheath and XO CrossÒ microcatheters.