UCI Urology (four Urologists), who specialize in kidney stone diseases include Dr. Pengbo Jiang, Dr. Jaime Landman, Dr. Roshan Patel, and Dr. Ralph V. Clayman, have teamed up with UCI CALIT2 engineers, Dr. William Mao, Dr. Mike Klopfer, and G. P. Li to design a new ureteroscope that is capable of removing all kidney stone fragments during surgery.
The team has six months to complete the project and has received a grant to develop the technology.
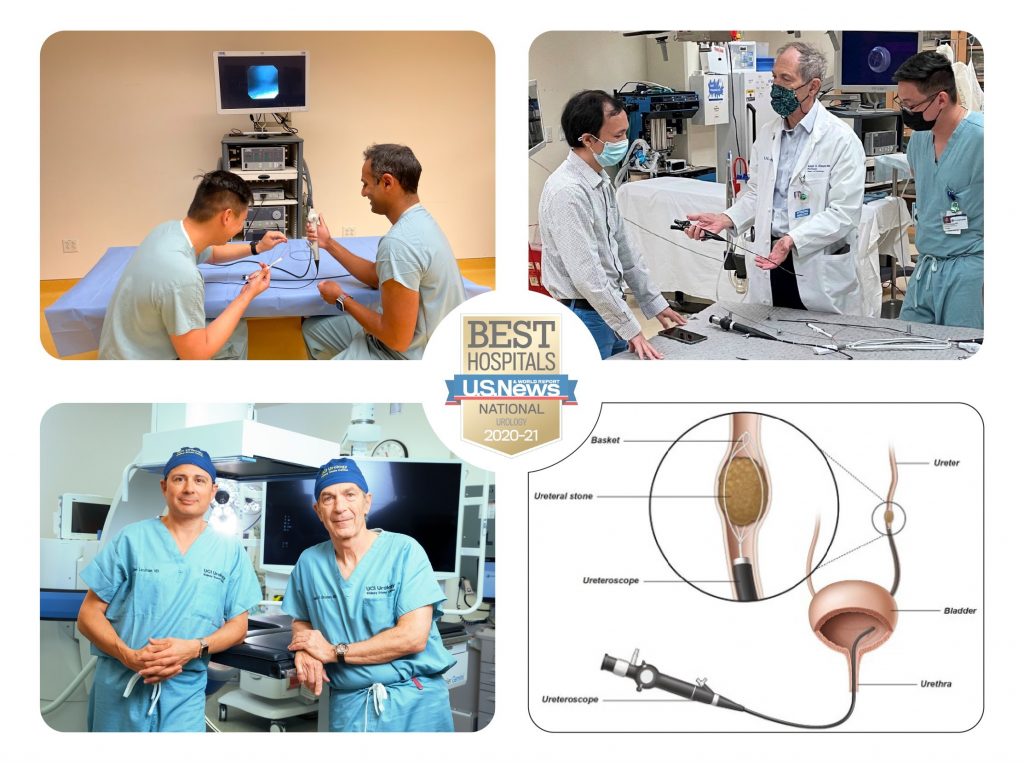
Approximately 10% of the U.S. population will experience a kidney stone. The primary treatment goals are to remove the stone with the least amount of patient discomfort and to prevent recurrence. One of the least invasive options for surgery involves using a non-incisional approach by entering the urinary tract with a ureteroscope. This type of endoscopic approach allows the urologist to access a stone and use a laser to break the stone into fragments.
Unfortunately, with the current technology, many of the smallest fragments cannot be recovered and removed. In fact, with the ureteroscope, even the best stone clearance rates are no better than 50 to 60%. The remaining fragments (about 2mm or smaller in size) can contribute to the formation of recurring stones within two years in as many as 40% of patients.
The UCI team seeks to develop instrumentation that is able to leave the kidney fragment-free following stone removal. The team’s proposal has been awarded a UCI Beall Applied Innovation Proof of Product (POP) grant, a funding program that accelerates commercially promising technology.
Dr. Ralph Clayman stated, “The POP grant has empowered and energized our work in this area. I am optimistic that given the great partnership we have with CALIT2 and the School of Engineering, within six months we will have a working prototype. This work has the potential to vastly improve surgical outcomes for all patients with kidney stones.”
The team has six months to complete this project and will utilize the Surgical Education and Research Center located at UCI Medical Center, Orange, CA. If successful, a disposable ureteroscope will be created that is able to remove all stone fragments, regardless of size. The ureteroscope will have additional features designed to improve the efficiency and effectiveness of stone removal, such as multiple laser channels and multidirectional deflection.
UCI Urology is at the forefront of research, providing patients with the best in innovative and less invasive urologic care. The department has a core mission to discover new advances in healthcare, teach tomorrow’s medical professionals, and heal patients with individualized care.