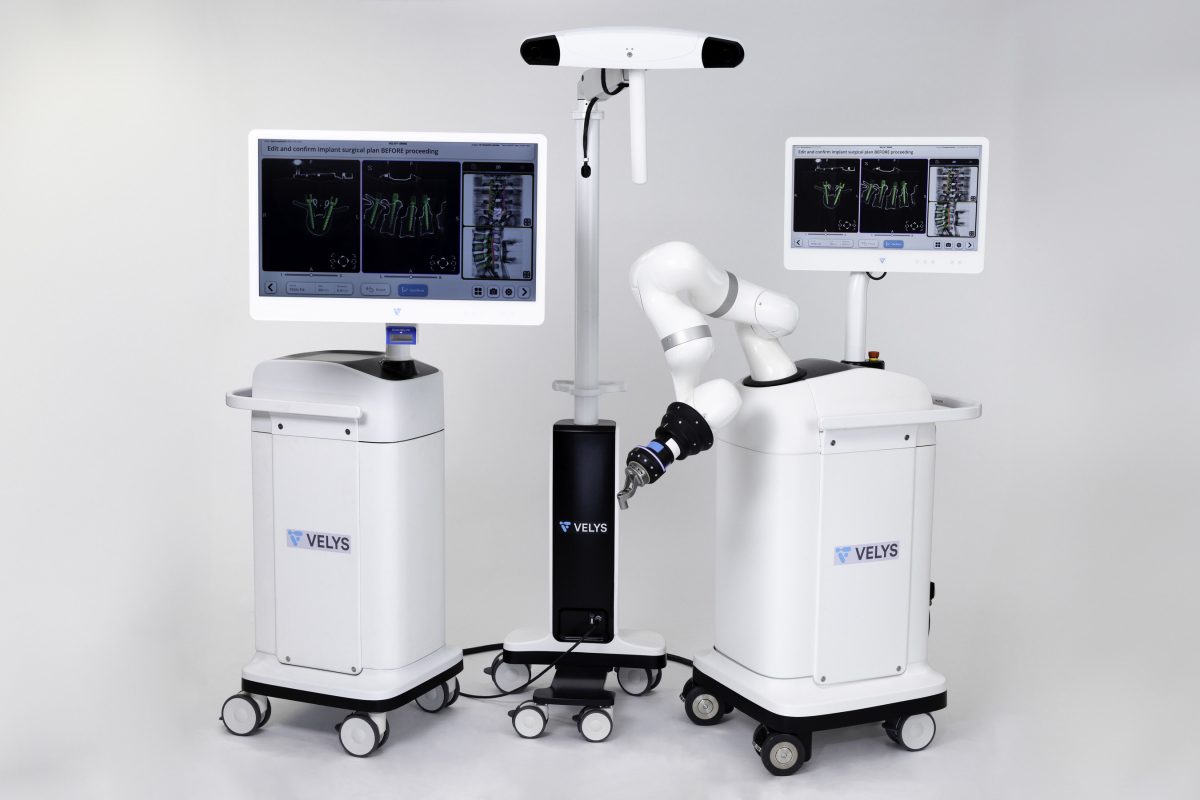
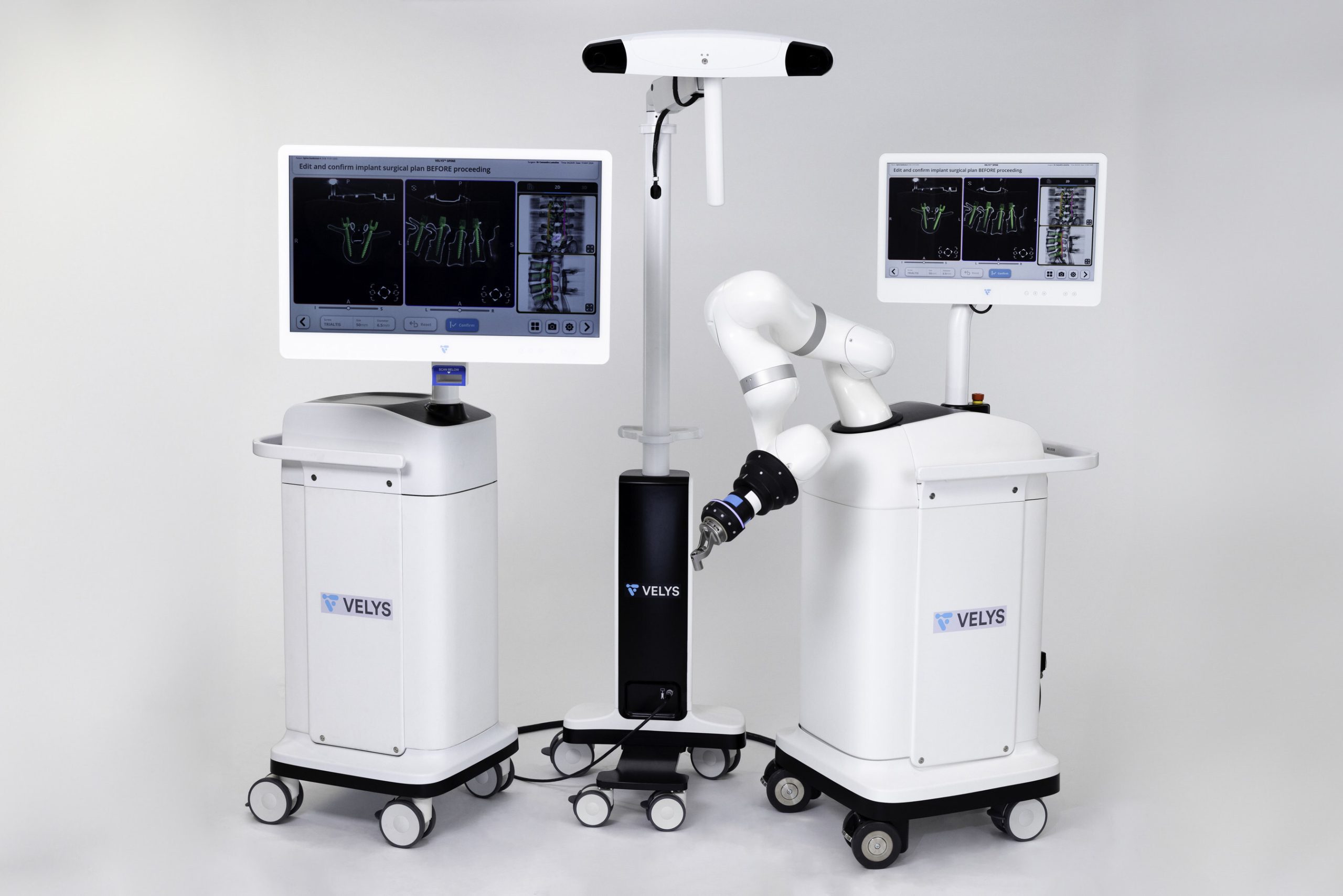
Today, Johnson & Johnson MedTech* announced that DePuy Synthes, The Orthopaedics Company of Johnson & Johnson**, is launching a proprietary dual-use robotics and standalone navigation platform developed in collaboration with eCential Robotics. The system, called the VELYS™ Active Robotic-Assisted System (VELYS™ SPINE), received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and is intended for use in planning and instrumenting spinal fusion procedures in the cervical, thoracolumbar and sacroiliac spine.
This new spine technology was designed to help surgeons tackle their most complex challenges. The VELYS SPINE system is dual-use, meaning it features both a standalone navigation and an active robotics platform that can enable surgeon flexibility in their approach and plans. Active robotics allows for surgical guidance tailored to surgeon preference. The distinctive features and capabilities of active robotics technology are set to establish a new standard in spine surgical care.
“We are shaping the next frontier of orthopaedic innovation with a relentless focus on digital advancements and excellence in the field of surgical robotics and navigation. Our dedication extends to enhancing patient care through significant strides in spine surgery,” said Aldo Denti***, Company Group Chair, DePuy Synthes. “This is a major step in growing our VELYS™ Portfolio and in our commitment to supporting spine surgeons and their patients with advanced tools.”
The VELYS SPINE system is designed to address the uniquely complex needs of spine surgeons; the system offers a customizable experience with pathology-specific workflows, aided by capabilities such as VELYS™ ADAPTIVE TRACKING TECHNOLOGY and VELYS™ Trajectory Assistance. It will be used with the DePuy Synthes core Spine portfolio of products, including the TriALTIS™ Spine System and Navigation Enabled Instruments, the SYMPHONY™ Occipito-Cervico-Thoracic (OCT) System, VIPER PRIME™ System and EXPEDIUM VERSE® Systems.
“Today’s landscape of enabling technologies features first-generation robotics systems that may face challenges in adapting to individual surgeon needs,” said Russell Powers†, Worldwide President, Spine, DePuy Synthes. “We recognize the urgent need for innovative solutions that offer new ways to engage with enabling technologies, returning control to surgeon’s hands. We believe that the unique features and capabilities of active robotics technology will set a new standard in surgical care for spine patients everywhere.”
Starting today, the VELYS™ SPINE system will be displayed throughout the U.S. in the MedTech Innovation Experience mobile lab, alongside other technologies and products currently available in DePuy Synthes’ comprehensive Spine portfolio. The MedTech Innovation Experience offers hands-on learning opportunities to surgeons, fellows, residents, and other healthcare professionals. Commercial availability is expected in the first half of 2025. Learn more about the VELYS™ SPINE system at www.ActiveRoboticAssistance.com.