VisionAir Solutions (VAS), the small company with a big idea for better therapeutic solutions, proudly announces a significant milestone of custom developing over 600 patient-specific airway stents using our AI VisionAir 3D Stent Software—helping save the lives of these patients.
This achievement marks the company’s commitment to bringing technological innovation to pulmonary medicine. Since its acquisition by Theken Companies last year, VAS has over doubled its growth and continues to demonstrate industry adoption and the advantages of personalized healthcare.
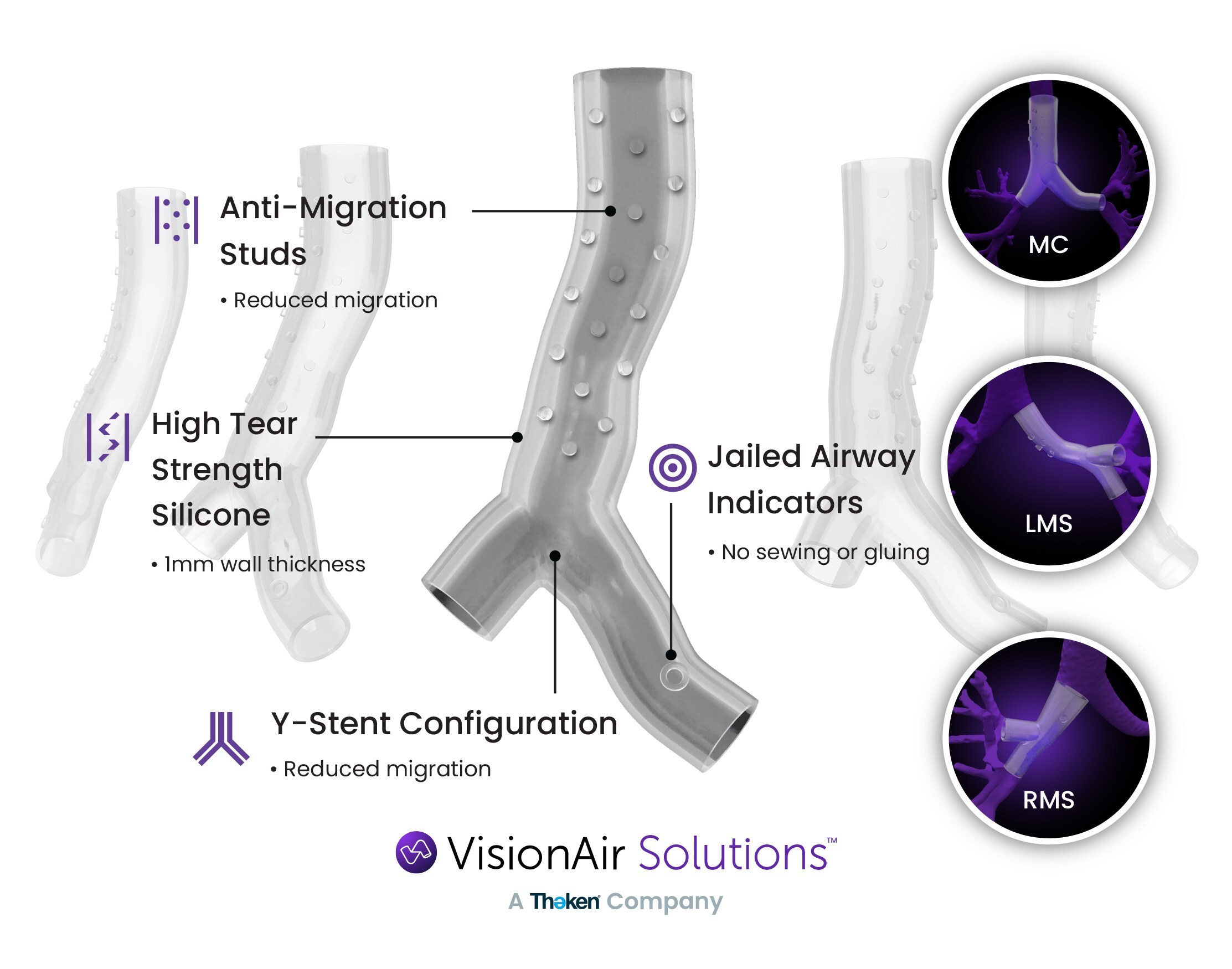
Utilizing its AI software and 3D printing technology, VAS has revolutionized the production of silicone airway stents—tailoring each implant to meet the unique anatomical requirements of individuals. These stents play a crucial role in enhancing respiratory function and improving the quality of life for patients with complex airway conditions; patients who were previously forced to surgically replace their “off the shelf” single lumen airway stent every 90 days can now receive a custom stent that fits their anatomy near to perfect, resulting in significant extended time before replacement.
“We are thrilled to reach this milestone,” said Randy Theken, Founder of Theken Companies. “It underscores our dedication to pushing the boundaries of what is possible in personalized medicine. Each stent represents a life-changing solution, designed by the physician and manufactured with anatomical precision, reflecting our ongoing commitment to collaboration, innovation, and patient-centered care.”
Additionally, VAS is excited to announce a strategic partnership with Lazzaro Medical. The aligned goals and innovations of both organizations revolve specifically around helping patients breathe easier. The partnership aims to advance the Tracheobronchomalacia (TBM) treatment options for both surgical and non-operative candidates. While the relationship is still in its infancy, the hope is to create industry-wide protocols for the evaluation and treatment of the millions of patients suffering from TBM.
As VAS continues to expand its 3D stent footprint and relationships, it is actively exploring new applications for its technology to include custom t-tubes and more readily available off-the-shelf patient matched airway stents (expected late 2024-early 2025).