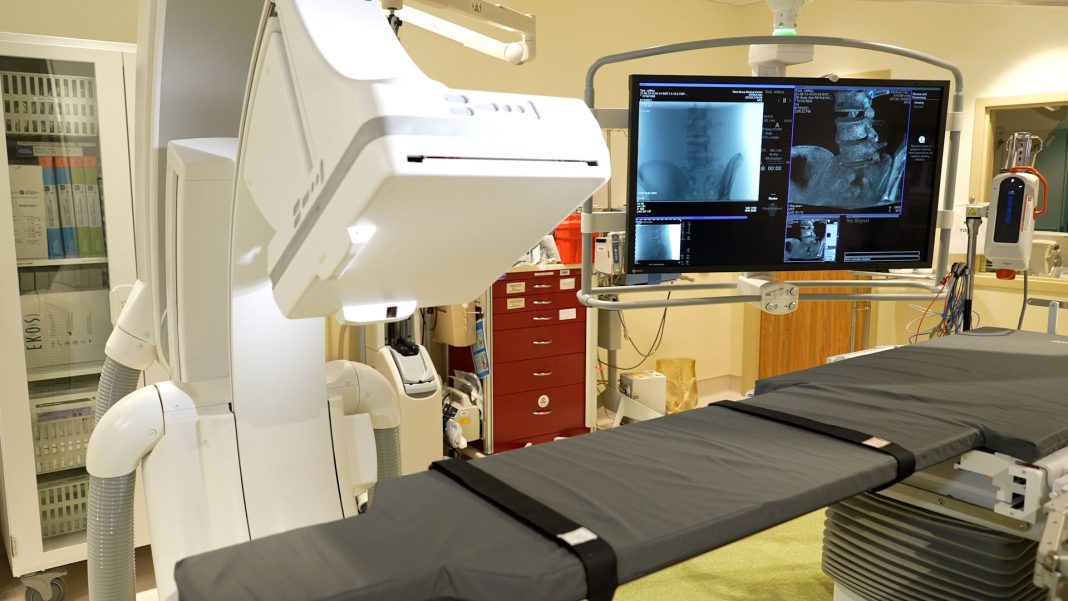
West Boca Medical Center increases its services in the community by offering patients advanced vascular and interventional imaging procedures in its new imaging suite and interventional radiology lab.
The new lab will add the Siemens Artis Q system to enhance imaging and invasive services. The system features high-resolution capabilities, which enhance minimally invasive therapies for peripheral vascular disease, embolization, and diagnostic examination.
“We are excited to bring this enhanced level of interventional and vascular capabilities to our patients that can be accessed right here in their community,” said George Rizzuto, chief executive officer of West Boca Medical Center. “Our first priority is to deliver the highest quality and safest treatments to our patients. These services and this new lab demonstrates our commitment by continuing to bring the most current technology and resources to Palm Beach County.”
Some of the benefits of this new technology include:
- Superior image quality that provides better visualization of small vessels, devices, and tissue.
- Enhanced system efficiency to produce the highest image quality at the lowest possible doses.
- Improved interventional imaging for treatment of peripheral vascular disease.
- Ability to perform rotational imaging for 3D reconstruction and vascular road mapping.
- Integration of other imaging modalities, including CT and ultrasound, that provides a basis for planning therapies, such as embolization of tumors.
This $4 million dollar investment will allow West Boca Medical Center to partner with sister hospital Delray Medical Center to expand its vascular services and offer patients this specialized care and technology in the community. For more information go to www.westbocamedctr.com or call 561-488-8000.