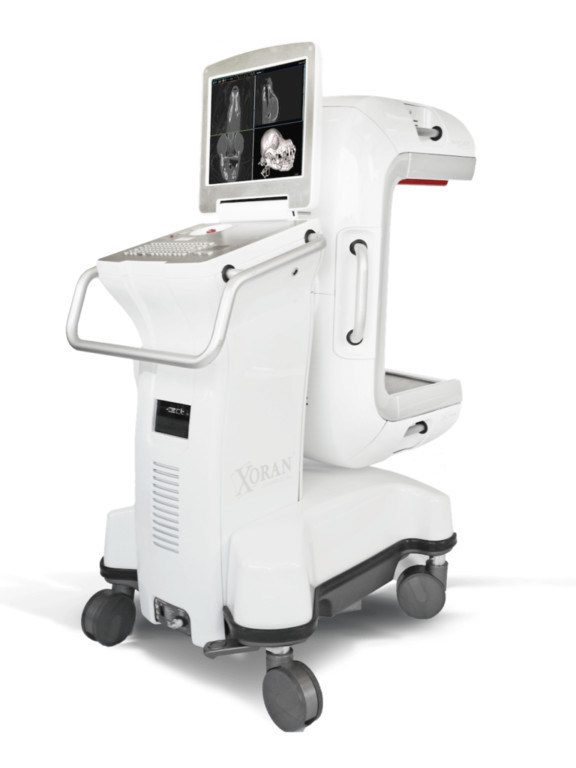
Xoran Technologies announced that the first VetCAT IQ™ has been installed at an exotics-only animal hospital. Chicago Exotics Animal Hospital, serving Chicago and the surrounding Illinois area, chose to implement the new VetCAT IQ™ model as it offers both bone and tissue imaging capabilities needed for the avian and exotic patient population.
The VetCAT IQ™—part of Xoran’s veterinary suite of products—is a compact, truly mobile 3D cone beam computed tomography (CBCT) system that brings imaging directly to the patient. Using Xoran’s advanced software viewing capabilities, veterinary specialists can diagnose and plan treatment in real-time, directly in the hospital location. Currently, VetCAT IQ™ is being used by veterinary dental specialists and ophthalmologists to view head, teeth, sinus, eye, and brain anatomy.
“With the addition of the VetCAT IQ™ at our clinic, we will help improve the lives of our most delicate creatures and significantly improve our ability to properly diagnose and treat every animal, not just the dog and cat community,” says Susan Horton, DVM, owner of Chicago Exotics. “The compactness of the VetCAT IQ™ is also a bonus. I was able to easily accommodate it into the floorplan of my imaging room.”
With the introduction of VetCAT IQ™ into an exotics only clinic, Chicago Exotics brings high-resolution 3D imaging capability to exotic animals of all kinds. Pets can be imaged during a fast, painless scan, and the image results can be reviewed by the clinician and the family right away.
“We couldn’t be more pleased to welcome Dr. Horton and the staff at the Chicago Exotics Animal Hospital into the Xoran family,” says David Sarment, DDS, President of Xoran and head of Xoran’s Veterinary Division. “This hospital joins an extended network of dental specialists, dermatologists, ophthalmologists, and generalists across the U.S. and Canada. We are excited to see the diagnostic images that Dr. Horton will be able to use further improve the care of her patients.”
Xoran is on a mission to partner with clinics and hospitals to equip veterinarians around the world with the same advanced imaging an image-viewing technology used in human medicine. Since 2001 Xoran Technologies has been the pioneer and medical market leader in point-of-care CT with over 1,000 installations worldwide. Leveraging this successful track record of 3D CBCT imaging, Xoran is passionate about applying their expertise to support veterinarians and improve animal healthcare.