ZAP Surgical Systems, Inc. today announced an agreement with Apollo Hospitals Enterprise Ltd. to acquire two ZAP-X® Gyroscopic Radiosurgery® systems. Apollo is well regarded as one of the most accomplished neurosurgery providers in India, and will become the first in South Asia to offer ZAP’s latest advance in completely non-invasive brain tumor treatments.
The agreement also aims to create a global radiosurgery center-of-excellence, namely “Apollo Brain Center,” dedicated to offering best-in-class technology and comprehensive neurosurgical care. First patient treatments with ZAP-X are expected to occur in early 2023 in New Delhi, followed by a subsequent installation in Mumbai.
Radiosurgery, also commonly referred to as SRS, is a well-established procedure for the non-invasive treatment of many primary and metastatic brain tumors, as well as select cancers of the head and neck. Often considered an alternative to costly and invasive surgical procedures, SRS is a non-invasive procedure that often provides equivalent to superior outcomes, yet requires no surgical incision, and little to no patient recovery period.
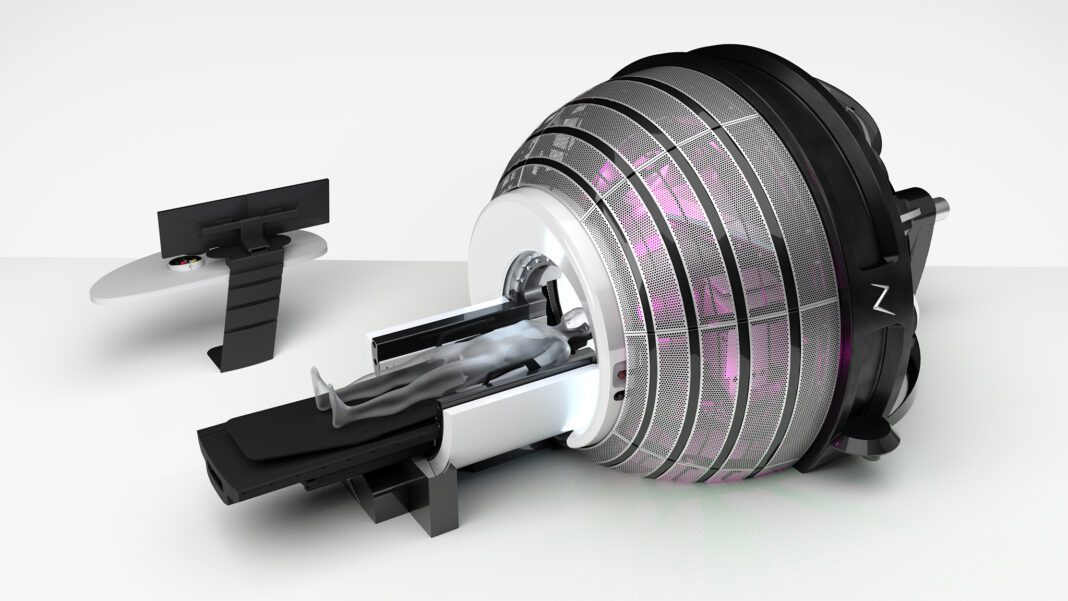
ZAP-X has transformed modern radiosurgery with a ground-breaking gyroscopic design that delivers hundreds of uniquely angled radiation beams. Combined, the myriad beams shape to the unique contours of targeted tumors with sub-millimetric precision. With this innovative approach, ZAP-X provides an enhanced ability to avoid critical structures and preserve healthy brain tissue. Additionally, where previous SRS technologies often required up to 5 patient treatments spread over multiple days, the high-precision of ZAP-X now enables many patients to be treated in a single outpatient procedure.
“With a special focus on radiosurgery for benign and malignant lesions as well as functional disorders, Apollo has always been the forerunner in introducing the latest in clinical technology to the region,” states Dr. Prathap Reddy, Founder and Chairman of Apollo Healthcare. “With intense research we zeroed in on ZAP-X based on its cutting-edge advantage over historical Cobalt-60 and robotic radiosurgery platforms. With further feedback from our neurosurgeons, we were confident that ZAP-X was the ideal technology to reinforce our standing as a best-in-class provider of neurosurgical care.”
ZAP-X is currently in clinical use at prominent institutions worldwide including Barrow Neurological Institute in Phoenix, Arizona, MedStar Georgetown Cancer Institute in Clinton, Maryland, the European Radiosurgery Center in Munich, Germany, and elsewhere. To date there are more than 30 additional ZAP-X system orders in various states of installation planning and commissioning.
“When treating the brain, especially with a complex procedure such as SRS, the tools must be highly specialized and impeccably precise,” says Dr. John R. Adler, CEO of ZAP Surgical and Emeritus Dorothy & TK Chan Professor of Neurosurgery and Radiation Oncology at Stanford University. “ZAP-X looks to set new standards in treatment quality and will be a significant asset in furthering Apollo’s commitment to excellence.”