ZEISS Medical Technology
ZEISS Medical Technology will showcase the latest in 3D visualization technology and surgical innovation at the American Society of Cataract and Refractive Surgery (ASCRS) conference from April 5-8, 2024, in Boston, MA. Building on its ZEISS Medical Ecosystem, several of the new innovations enable seamless data integration and management across the cataract and refractive workflows, creating a new standard for efficient, personalized ophthalmic care.
Euan S. Thomson, Ph.D., President of Ophthalmology and Head of the Digital Business Unit for ZEISS Medical Technology
“ZEISS continues to redefine the digital ophthalmic market, reimagining diagnostic and surgical workflows to include ground-breaking 3D visualization solutions. We’re consistently innovating to deliver on our vision for the future of ophthalmology through fully connected, digital workflows that are setting new standards for efficiency and patient care to meet the evolving needs of ophthalmic surgeons around the world.”
The future of 3D visualization in ophthalmic surgery
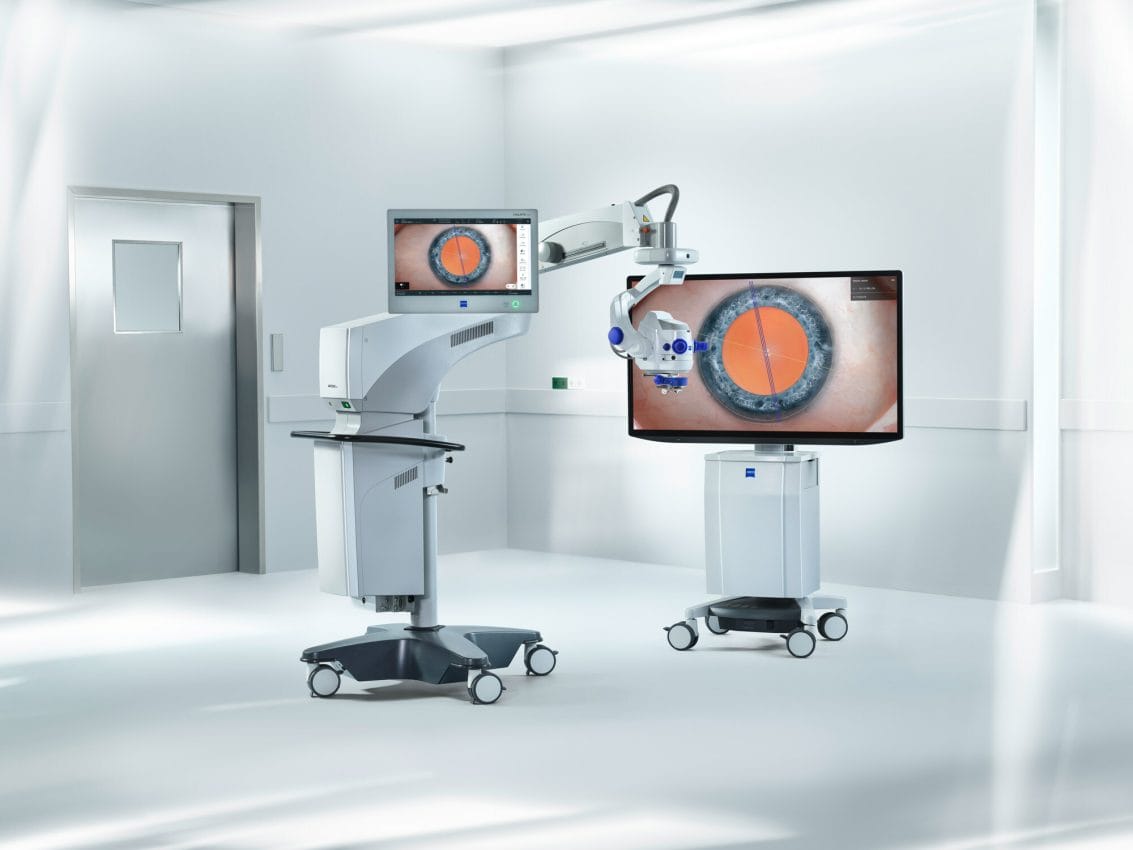
ZEISS is extending its workflow offerings with new surgical and visualization technologies at ASCRS. The ARTEVO® 850 3D heads-up ophthalmic microscope from ZEISS, used in both anterior and posterior surgical cases, is setting the pace in digital visualization with true color imaging thanks to the HDR monitor and two 4K 3-chip cameras, allowing the surgical field to be displayed in high resolution. Leveraging the expanded depth of field available with the new Smart DoF (Depth of Field) setting, surgeons may increase depth of field by nearly 60 percent.2 Additionally, the ZEISS ARTEVO 850 with CALLISTO eye® features a redesigned intuitive user interface that centralizes all controls on a single touchscreen.
ZEISS is also showcasing the new ARTEVO® 750, the latest addition to ZEISS’s portfolio of optical surgical microscopes, which elevates the surgical workflow by introducing advanced optical visualization technology including new RGB LED illumination with adjustable light color temperature. The well-established Stereo Coaxial Illumination (SCI) delivers highly stable and high contrast red reflex during surgery. Utilizing the AdVision®, data is displayed in the eyepiece with a 40 percent increase in resolution.3 The re-designed user interface of ZEISS CALLISTO eye provides a central point of control facilitating access to patient management and cataract assistance functions.
Digital integration of the ZEISS ARTEVO 850 and 750 into the ZEISS Cataract Workflow allows seamless data transfer from the ZEISS IOLMaster 700, ZEISS EQ Workplace and ZEISS VERACITY Surgery Planner.4 In addition, post-surgery review, analysis and sharing of surgery videos is now made possible with the ZEISS Surgery Optimizer featuring AI-based video segmentation.
At ASCRS, a new spatial computing app for reviewing 3D videos, based on the ZEISS Surgery Optimizer, will be demonstrated as an exclusive experience on Apple Vision Pro. This app represents an entirely new way for ophthalmologists to review 3D videos pre-recorded with ZEISS digital surgical microscopes. Using an Apple Vision Pro to review surgical videos like never before, surgeons can easily and simultaneously view 2D or 3D surgical videos, scans of the eye, patient information and much more. In addition to reviewing videos, ZEISS IOLMaster 700 scans and biometry results are also conveniently viewable next to 3D videos, as well as refraction results, to provide the surgeon with a comprehensive view and experience, easily controlled through Apple Vision Pro’s three-dimensional user interface controlled by a user’s eyes, hands and voice.
“As ophthalmologists, precision is everything,” said Dr. Tommy Korn, MD, Ophthalmologist and Chief Physician Evangelist for Digital Health Innovations at Sharp HealthCare in San Diego, CA. “We’ve long relied on 2D images to guide us, but with the ZEISS Surgery Optimizer application for the Apple Vision Pro, ZEISS redefines what is possible when viewing stereoscopic eye surgery videos. Viewing eye surgeries in 3D is like being there in the operating room—every detail, every movement is real. It’s a game changer for me as a surgeon. Once you go 3D, you can’t go back.”
The ZEISS Surgery Optimizer application for the Apple Vision Pro is available for download from the Apple App Store here.