For years, surgeons performing Cotton and Evans osteotomies have navigated a narrow choice set: allograft wedges with inherent variability and supply-chain risks, or metal implants that may require removal and cannot biointegrate. NovaBone’s Bioactive Precontoured Wedges (BPW) introduce a third path, one that corrects deformity while actively accelerating the body’s own healing process.
Built on scientifically credible biomaterials and engineered with a precontoured, anatomical shape for Cotton and Evans procedures, BPW is the first-to-market precontoured, bioactive glass (45S5) wedge regulated by the FDA, as a Class II device. The result is a unique combination of structural support, osteostimulation, and full resorbability, offering surgeons and patients a new standard for superior reconstructive outcomes.
The Unmet Need: Stability, Reliability, and True Integration
Osteotomies are precise, load-bearing corrections. Traditional solutions require surgeons to compromise:
- Allografts bring variability in geometry and quality, logistical constraints, and the potential for disease transmission risk associated with donor tissue.
- Metal implants provide short- and long-term structural integrity, but do not remodel into bone and can require secondary surgery for removal, adding cost, risk, and patient burden.
- Autograft options, while biologically compatible, are limited in volume and can, on occasion, introduce donor site morbidity.
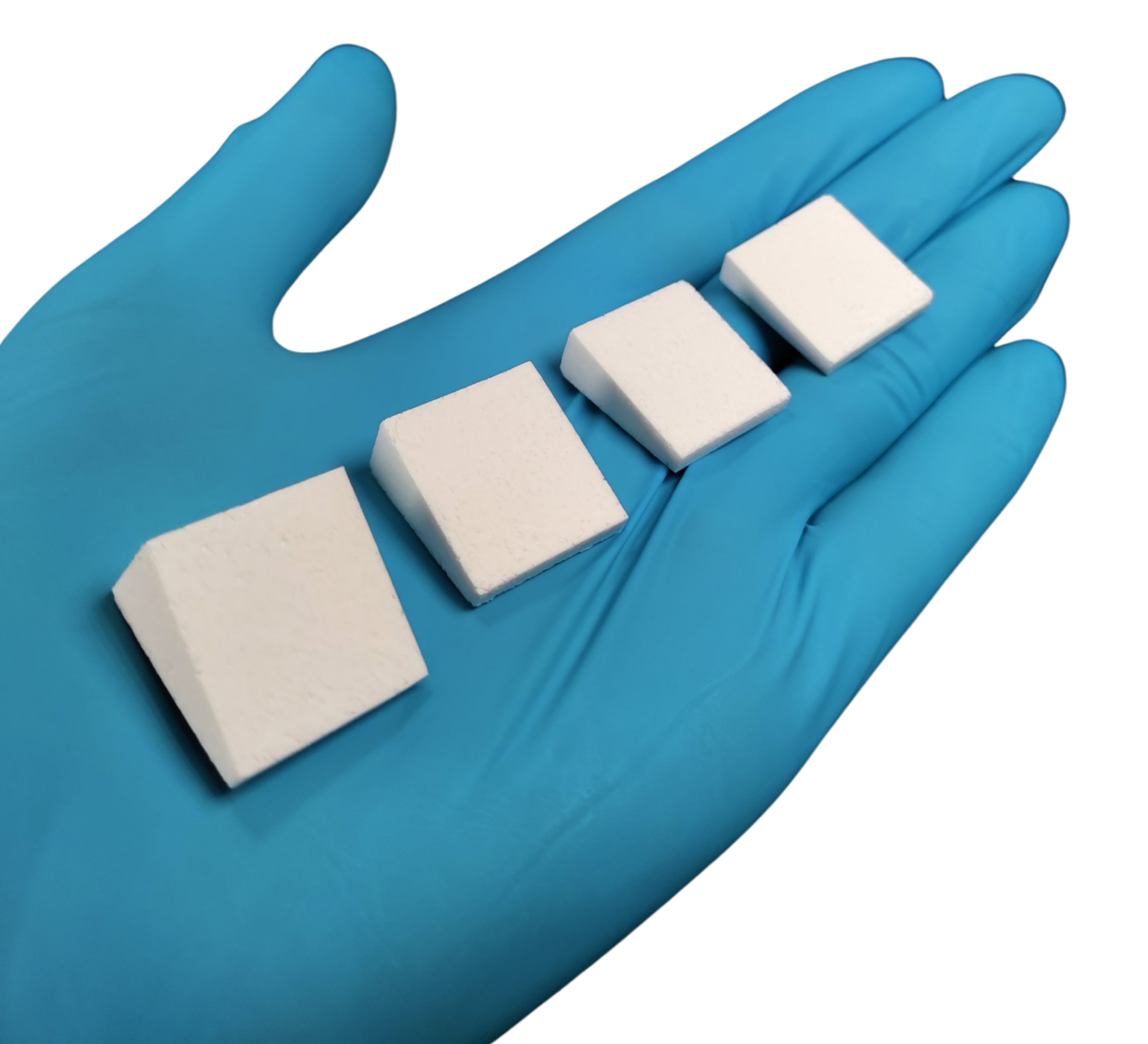 Bioactive Precontoured Wedges (BPW) was designed to close these gaps with a single, surgeon-ready device that combines anatomical precision, structure, and integration, thus delivering the stability of a wedge today and the promise of native bone tomorrow.
Bioactive Precontoured Wedges (BPW) was designed to close these gaps with a single, surgeon-ready device that combines anatomical precision, structure, and integration, thus delivering the stability of a wedge today and the promise of native bone tomorrow.
Why Bioactive Glass? And Why Now?
Bioactive glass 45S5 has, for decades, established a robust clinical and scientific heritage. As a synthetic calcium phospho-silicate, it is valued for its handling characteristics, osteoconductivity, and a surface reactivity that supports osteostimulation. When configured as a porous, interconnected scaffold, it does more than occupy space: it readily mineralizes, recruits osteoblasts as a catalyst for new bone formation, and is ultimately replaced by the patient’s own bone. In short, it is a material that participates in healing.
BPW leverages this science in a precontoured wedge tailored to the geometric and biomechanical realities of Cotton and Evans osteotomies. The device is pre-operatively hydrated (sterile saline, patient’s blood), can be customized intraoperatively with standard instruments, and is compatible with traditional metal plating/fixation systems and innovative non-metal staple solutions.
What Makes BPW Different
BPW isn’t a repurposed graft or a generic block; it’s purpose-built for Cotton and Evans procedures. Several attributes differentiate it from alternatives:
- Precontoured, anatomical fit: Designed for efficient surgical workflow and reliable placement.
- Bioactive architecture: An interconnected, porous structure enables rapid mineralization and new bone ingrowth.
- Resorbable scaffold: BPW is fully resorbable and intended to be replaced by native bone.
- Strength where it counts: BPW exceeds the compressive strength of allograft, providing confidence in early structural support while the body remodels.
- Device classification, though, not metal: A non-metal wedge, regulated as a Class II device, focused on patient safety and intra-operative effectiveness.
These features converge on a single, simple idea: offer surgeons a wedge that behaves like an implant in the OR and then hand the baton to biology, so the construct becomes living bone.
Evidence Snapshot: Mechanical Performance and Early Clinical Experience
Independent of marketing claims, BPW has been evaluated for both mechanical performance and clinical outcomes in real-world, reconstructive scenarios. A recent AOFAS abstract submission examined how porous bioactive wedges behave under simulated physiological conditions and how patients performed following deformity correction procedures that included Cotton and/or Evans osteotomies.
In vitro findings:
- Predictable resorption profile: Mass loss and compressive strength loss progressed approximately linearly over the evaluation period, consistent with an orderly transition from device to bone.
- Mechanical integrity across time points: Under dynamic loading representative of physiological stress, wedges completed the loading cycles, supporting the intended role as a stability-providing scaffold during healing.
Clinical observations (retrospective experience; general summary level):
- Patients undergoing reconstruction with BPW demonstrated radiographic improvement and reported functional progress in follow-up.
- Across the reported cases, there were no adverse events or implant failures during the observation period.
Complementing the abstract, a retrospective case study authored by Dr. Charles Cook, MD, underscores the importance of a pre-contoured shape that supports efficient workflow and can provide a safe, effective scaffold for new bone growth, helping ensure surgical site stability, and minimizing graft failure risk. While additional, larger-scale studies are warranted, these early results align with the expected behavior of a bioactive, porous, resorbable wedge and support its role as a reliable, void-filling scaffold in osteotomies.
Translating Science Into Surgical Value
For the surgeon, clinical value is created at the intersection of handling, reproducibility, and biological performance:
- Handling & efficiency: The precontoured geometry reduces shaping time, while ready fluid uptake and standard instrument customization fit existing workflows.
- Stability through the healing arc: The strength profile supports early stability; the interconnected porosity supports new bone formation; the resorption allows a transition to native bone aligned to the timeline of osseous healing.
- Compatibility: BPW plays well with plating and staple solutions, preserving surgeon choice and enabling construct designs that reflect surgeon preference and anatomical intuition.
Patient-Centered Advantages
Patients want to heal once, heal well, and return to life. BPW supports those goals:
- No permanent hardware: As a fully resorbable construct, BPW removes the prospect of implant removal, reducing the burden of secondary procedures.
- Biology-first healing: The wedge is designed to become bone, supporting functional progress reported in early experience and radiographic improvements aligned with correction goals.
- Safety profile: In the aforementioned Cook series, no adverse events or implant failures were reported over the follow-up period.
Executive & Investor Lens: Resilience, Cost, and Adoption
Medical Device News Magazine serves an audience that must weigh clinical promise against operational reality. BPW’s differentiation extends beyond the OR:
- Manufactured in the USA: Domestic manufacturing offers supply-chain resilience, reduced tariff exposure, and a practical answer to global lead-time volatility, a very tangible benefit for IDNs, GPOs, and procurement teams.
- Five-year shelf life: A long shelf-life eases inventory planning, simplifies stocking across multi-site systems, and reduces expirations, which are important for IDNs and independent ASCs.
- More cost-effective than metal implants: By providing a non-metal, resorbable alternative with bioactive benefits, BPW brings an economic and clinical rationale that can successfully pass value analysis committee review.
- Platform heritage: NovaBone’s bioactive glass technology comes with 20+ years of clinical use and more than two million devices implanted across all orthopedic segments.
For investors and strategics, the signal is clear: BPW sits at the intersection of material science, device engineering, and surgeon-centric design.
Engineering Notes: Designed for Precision, Built for Bone
Several design choices deserve emphasis for the technical reader:
- Porosity tuned for biology: The interconnected porous network is engineered to enable rapid mineralization and osseous ingrowth, supporting the cascade from initial scaffold to native bone.
- Pre-hydration readiness: The construct readily absorbs fluid of the surgeon’s choice, promoting handling and potential cellular interaction at the interface.
- Intraoperative flexibility: While precontoured, the wedge can be fine-tuned with standard instruments to accommodate anatomical nuance, critical in revisions or complex deformity.
- Strength where needed: The 2.5× compressive strength vs. allograft claim provides confidence in initial support, while resorption aligns the long-term outcome with living tissue rather than permanent hardware.
Surgeon Workflow & Standardization
Efficiency and consistency are the cornerstones of surgical quality. BPW’s precontoured fit and compatibility with existing fixation can streamline steps, reduce intraoperative decision fatigue, and support repeatable outcomes across teams and sites. For training programs and multi-hospital systems, such standardization can be an enabler for quality initiatives and time-in-OR optimization.
Safety, Efficacy, and the Evidence Curve
As with any new device class, evidence matures over time. The current body of work in vitro mechanical performance, dynamic loading under physiologic conditions, and retrospective clinical experience reporting radiographic improvement, functional progress, and no adverse events or implant failures providing a confident early signal. The logical next steps include larger prospective studies, longitudinal follow-up, and broader site adoption to validate and refine indications. NovaBone’s approach emphasizes science-driven iteration, refining devices with surgeon feedback and clinical data to support real-world performance.
Supply Chain & Procurement: Practical Advantages
In today’s environment, availability and logistics can determine whether a promising new technology gets used. BPW’s combination of U.S. manufacturing and five-year shelf life reduces stock-out risk and simplifies distribution planning. For procurement professionals, the device’s non-biologic, fully synthetic nature removes the variability intrinsic to allograft sourcing and sidesteps cumbersome donor screening and tissue bank dependencies. For systems managing diverse surgical volumes, those realities translate into fewer surprises and cleaner forecasting.
Voices from the Field
Clinicians evaluating BPW have highlighted the precontoured geometry and workflow advantages as tangible benefits. As Dr. Charles Cook, MD, notes, the wedge’s shape and bioactive scaffold properties can support surgical stability and minimize graft failure risk – all key drivers of both outcomes and surgeon confidence.
Readiness for Real-World Integration
Because BPW is a device regulated as a Class II device, and because it is compatible with plating and staples, surgeons can integrate it without overhauling their construct philosophy. The learning curve focuses on handling characteristics: pre-hydration, contour confirmation, and, when desired, minor customization with standard instrumentation. For hospital administrators and service line leaders, that means adoption without disruption.
Looking Forward: Where Bioactivity Meets Design
The arrival of BPW is a milestone but also a beginning. As more centers integrate bioactive, resorbable wedges into deformity correction and elective reconstruction cases, expect a growing body of evidence on time to incorporation, radiographic consolidation, functional recovery, and revision rates. NovaBone’s innovation roadmap remains anchored in surgeon-centric design and evidence-based improvement, expanding where bioactive scaffolds can replace static implants and give synthetic bone graft substitutes the final word.
Conclusion: A New Standard for Cotton and Evans
NovaBone’s Bioactive Precontoured Wedges offer a compelling answer to a longstanding challenge in foot and ankle reconstruction: how to stabilize today’s correction while building tomorrow’s bone. By uniting precontoured precision, bioactive porosity, resorbability, and device-level reliability, BPW provides surgeons with a wedge that works in the OR and wins with accelerated healing. With U.S. manufacturing, a five-year shelf life, cost advantages versus metal, and compatibility with standard fixation, the device is engineered not just for clinical performance, but for real-world adoption in hospitals and ASCs.
For administrators, engineers, clinicians, procurement professionals, and investors, the signal is consistent: bioactive, resorbable, precontoured wedges are no longer an aspiration; they are here, bringing together science, design, and practicality to elevate outcomes in elective osteotomies… and beyond.
Note: BPW is regulated as a Class II device. Early clinical experience summarized above reflects retrospective observations and an AOFAS abstract submission reporting radiographic improvement, functional progress, and no adverse events or implant failures over the reported follow-up period. Larger studies are anticipated as adoption expands.
Editor’s Note: Scott Day is Vice President of Business Development at NovaBone, a global leader in bioactive glass technology for bone and wound healing. With over 20 years of experience driving growth across the medical device and orthobiologics industries, Scott brings deep commercial expertise and a passion for innovation to his work. His career spans leadership roles at both startups and established companies, where he has led go-to-market strategy, global OEM partnerships, and transformative sales growth. At NovaBone, Scott is focused on expanding global OEM and private label relationships—delivering strategic insights in sales, clinical, and regulatory domains to ensure partners succeed in competitive therapeutic markets.
