Spyros Achinas1, Efthymios Poulios2, Nikolaos Chralampogiannis3
1 Regenity Biosciences, Blauwborgje 32, 9747AC, Groningen, The Netherlands
2 4th Department of Surgery, Attikon University Hospital, National and Kapodistrian University of Athens, Medical School, Rimini 1, Chaidari 124 62, Athens, Greece
3 Department of Urology, Faculty of Medicine, Heidelberg University, Ludolf-Krehl-Straße 13-17, 68167 Mannheim, Germany
The use of computer systems has been considered a strategically distinctive option for the entire transition to digital data management. The gap between the paper-based and the electronic records calls into question how the MedTech industry can ensure the compliant data management. The computer systems enable granular visibility of the enterprise operations and insights beyond the production floor and efficiently adapts to computing requirement peaks and valleys.
Subtle issues are popped up amid the validation of electronic data generating systems which may underpin the drive for globally modernization and digital transformation in MedTech. The quest for devising a suitable means of smart validation has been a matter of concern of validation engineers (See fig. 1 for pivotal aspects of CSV). Over the last years, MedTech industry has progressively integrated computerized systems to support production. As a consequence, their criticality on GMP level has increased the pressure from regulatory authorities on clarification of the computers’ role on industry. Regulatory audits, related to computerized systems validation, generate significant observations on the aspects of qualification and data integrity. These observations can lead to serious caveats of the applied manufacturing and quality system with direct consequences on the business and corporate image.
Business leaders envision the implementation of computerized systems in MedTech industry as a significant aspect to create a healthy economic arena; thus, digitised approaches to reduce time is indispensable to re-direct the linear economy into a sustainable trajectory. The leapfrogging to computerized systems and their integration for automated manufacturing processes can have a positive contribution to the overall production savings and may create a roadmap to establish attractive economic activities. In the past, one of the criticisms levied at the implementation of computerized systems has been the lack of a standardized policies and guidelines. EU GMP Annex 11 and US FDA 21 CFR Part 11 are nevertheless pivotal incentives for the manufacturers to accelerate digital transformation providing transparency, productivity and agility across the enterprise.
Building a Solid CSV Foundation Will Improve Execution and Efficiency
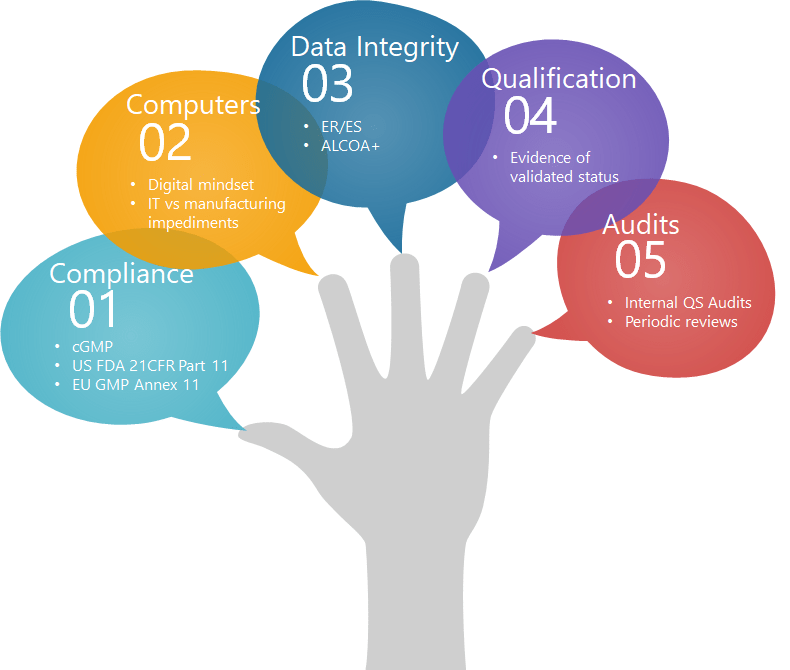
Figure 1. Enumeration of essential factors for CSV.
Bridging computational requirements with manufacturing factors assertively tacks tensions and trade-offs at the technological-policy interface and revives competitiveness. Industrial initiatives are recommended by the European Union to enhance the computer systems sovereignty and to add industrial credibility and benefits for the society. Current practices and policies for computer validation are not coherent, and more synergy and dedication towards a solid CSV foundation would ease the integration of computerized systems. While computer systems are built by the software companies based on the CSV regulations, MedTech manufacturers must revalidate the computerized systems in their facility to maintain the validated status. The regulatory bodies during inspections expect to see documented evidence of technical approach for validation and data management. Strong evidence of validation will lessen the risk to patients and purvey rational safeguards against hurdles in clinical practice.
The generation and manipulation of the vast amount of data is critical for business leaders. A holistic approach to leverage digitalization and engineering facets is essential to interconnect all participants in the product lifecycle – operators, buildings, machines, materials, engineers , designers and automation, products. Adopting digital mindset and enabling smart engineering are intrinsic for the transition towards sustainable business processes. Data integrity is regarded as the sine qua non of successful commercialization and delivery of medical devices, thus, CSV concept is regarded as the centrepiece of departmental consolidation and enterprise realignment. The CSV concept manifests an evolving scheme that is characterized by a patient-centricity, , veritable brainstorm attitude, inaugural global engagement, upfront technological requisite and an apparent economic arena.
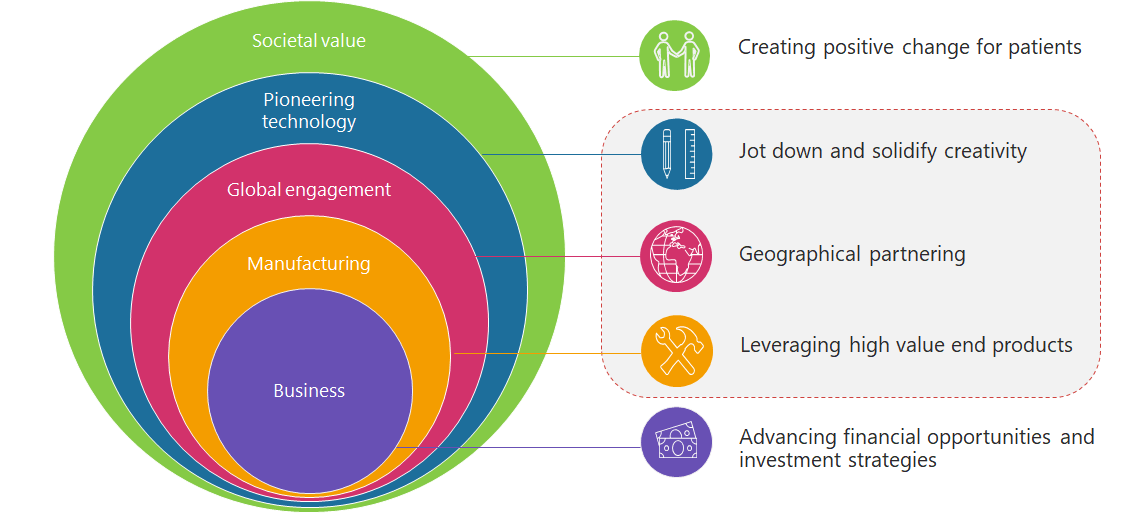
Figure 2. Scheme of aspects contiguity in MedTech enterprises. Grey area depicts the potency wherein CSV can be implemented.
This article proffers a brief abridgment on the standpoints of CSV implementation in the MedTech industry.
References
- https://us.hso.com/blog/the-future-of-software-validation-in-pharmaceutical-life-sciences-medical-device-manufacturing-organizations/
- https://pflb.us/blog/computer-system-validation-benefits/
- https://biotech.com/2019/11/06/how-computer-systems-validation-can-make-or-break-your-business/
Spyros Achinas is validation engineer at Regenity Biosciences in Groningen, the Netherlands. He started his career as bioprocess development engineer at the Bavarian Institute for Agriculture in Munich where he worked in the development of fermentation pilot units. Later, he worked as research engineer at the University of Groningen where he worked on the design and fabrication of 3D printed bioreactors. Spyros held roles in production and validation at Thermofisher Scientific in Groningen. Spyros has B.S/M.S in Chemical Engineering and M.S in Automation Systems from the National Technical University of Athens and M.S. in Sustainable Resource Management from Technical University of Munich.
Dr. Euthymios Poulios obtained his medical degree at the Medical School of the University of Athens in 1999. He completed his specialty in General Surgery at the Constantopoulio Hospital of Nea Ionia “Agia Olga” in 2008, participating and performing more than 800 operations. He retrained in advanced laparoscopic surgery and minimally invasive surgery at the University of San Francisco and the University of Strasbourg. He specialized in advanced colon surgery at Cleveland Clinic and Robotic surgery at Yonsei University. In 2009, Mr. Poulios joined the 1st Surgery Clinic of the Hygeia Hospital.
Dr. Charalampogiannis completed the specialty of Urology at the Red Cross Hospital of Athens in 2011. From October 2012 to December 2015 he was appointed as Consultant Urologist at Diakonie Krankenhaus of Schwäbisch Hall, Germany. From January 2016 to September 2019 he worked as Consultant at the Urologic Department in SLK-Kliniken am Gesundbrunnen, in Heilbronn, Germany. He’s been in private practice in Öhringen, Germany since 2019. From April 2019, he is PhD candidate at the Faculty of Medicine at University of Heidelberg focusing on the use of a robot for treatment of complicated nephrolithiasis. He has authored more than 30 full text manuscripts published in peer-reviewed journals.