October 12, 2020
ADEXUSDx® COVID-19 antibody test update: Now Diagnostics announced that an independent validation study of the ADEXUSDx® COVID-19 antibody test conducted by the University Laboratory of Clinical Microbiology, Virology and Bioemergency Diagnostics at the Luigi Sacco University Hospital, University of Milan has found the ADEXUSDx® COVID-19 antibody test has 100% specificity and 94.3% sensitivity, meeting the requirements for clinical use, offering patients fast and highly reliable results.
The clinical evaluation, conducted on 300 samples, has proven the exceptional performance of the innovative diagnostic device, which received a Conformité Européene (CE) mark for use in moderate complex testing settings across 28 countries in the European Union (EU) in June 2020. With a CE marking, C19 Development, LLC, a wholly-owned subsidiary of NOWDiagnostics, has begun offering the ADEXUSDx® COVID-19 antibody test for use in a variety of health care settings in the EU—from clinics to hospital emergency rooms, while launching clinical trials of the test for use at point-of-care and over-the-counter.
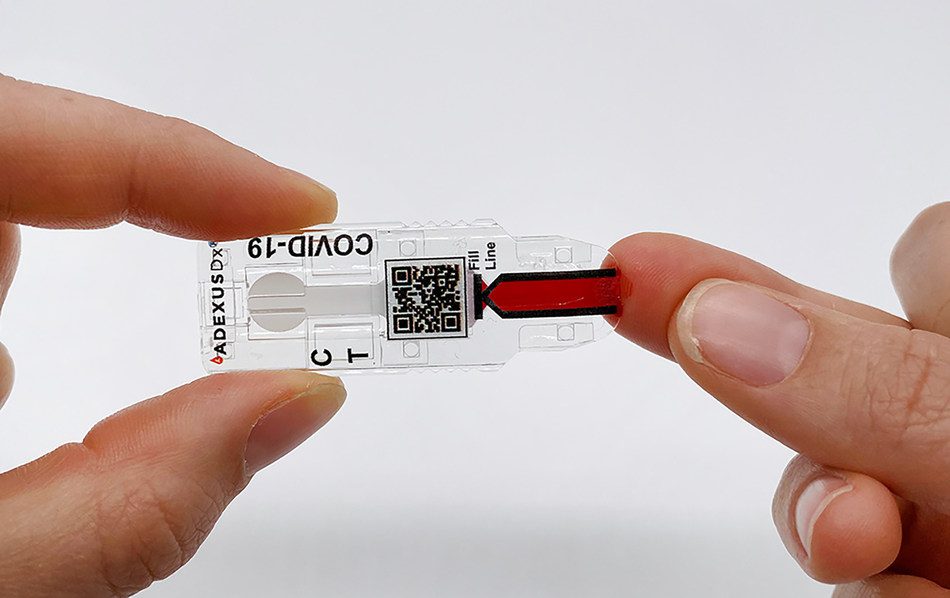
The ADEXUSDx® COVID-19 Test uses an FDA-cleared and CE-marked platform containing next-generation, easy-to-use technology that is affordable, portable, and delivers laboratory-quality results in minutes without any additional supplies. Employing sophisticated separation microfluidics and proprietary multi-layer membranes, the ADEXUSDx® COVID-19 Test separates plasma from whole blood, automatically controlling the sample volume allowing for highly accurate antibody testing and, in minutes, displays results indicating whether the subject has developed SARS-CoV-2 antibodies for COVID-19. This can include those who have been infected with the virus, regardless of whether they presented with severe, moderate, mild, or no symptoms, as well as those who have cleared infection.
The University of Milan validation study found that an important advantage of the ADEXUSDx® COVID-19 antibody test is that it detects three classes of antibodies – IgA, IgG, and IgM – present in the bloodstream. The study credits the addition of IgA, generally produced early by the body during infections, for the ADEXUSDx® COVID-19 Test’s utility for early identification of subjects who have developed an adaptive immune response to SARS-COV-2.
The ADEXUSDx® COVID-19 Test is a rapid serology, a self-contained assay that measures the presence of SARS-CoV-2 antibodies to deliver accurate and reliable results in 15 minutes with no buffers, reagents, or additional equipment or supplies. The ADEXUSDx® COVID-19 Test has demonstrated a high level of analytical performance with 95.6% sensitivity (true positive rate) and 98.5% specificity (true negative rate). NOWDiagnostics submitted an application to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the test on May 29, 2020. The application is still pending.