October 29, 2020
Advanced Chemotherapy Technologies, Inc., is a clinical-stage drug delivery company. Today they announced they closed a $5.5 million Series A investment round led by Khosla Ventures.
Advanced Chemotherapy Technologies notes the capital will be used to fund initial clinical development of the company’s ACT-IOP-003 local chemotherapy system for the treatment of locally advanced non-resectable and borderline resectable pancreatic cancer.
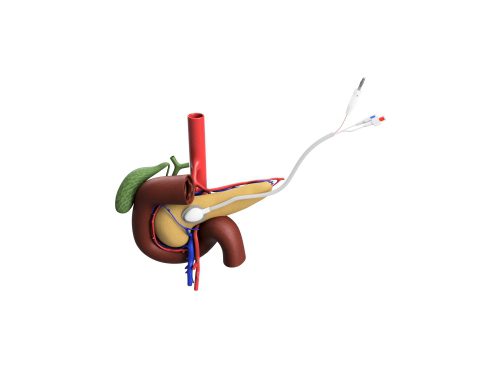
For pancreatic cancer, the ACT-IOP-003 system will be used to deliver the chemotherapy drug, gemcitabine, through the dense tumor microenvironment, directly to the tumor, while also minimizing the systemic toxicity commonly associated with chemotherapy treatments for pancreatic cancer. This approach offers three major advantages over traditional systemic chemotherapy: (1) Superior delivery of chemotherapy to the tumor cells, greatly increasing the amount of drug to treat the tumor, (2) Tumor shrinkage that can enable surgical resection, the only curative treatment for pancreatic cancer, and (3) Greatly decreased systemic toxicity so that the patient can remain in treatment.
This novel implantable drug delivery system uses a mild electrical current (iontophoresis) and can deliver a wide range of drugs directly to the local tumor. The system was developed in the laboratories of Jen Jen Yeh, MD, and Joseph M. DeSimone, PhD, at the University of North Carolina (UNC) at Chapel Hill. In preclinical studies, 100% of pancreatic cancer tumors treated with the device using gemcitabine shrunk by an average of 40%, while tumors treated with intraveneously delivered gemcitabine grew an average of 240%.
“ACT has made remarkable progress leveraging their seed investment and NIH grants to advance their first product, ACT-IOP-003, into the clinic,” said Vinod Khosla, founder of Khosla Ventures. “ACT brings a skilled team and unique approach to drug delivery and precision medicine and we are excited to build on this momentum.”
“We are excited to have the support of such an outstanding Venture Capital firm in Khosla Ventures,” said Tony Voiers, CEO of Advanced Chemotherapy Technologies. “We are now poised to move our lead product into the clinic, where we will have the opportunity to treat pancreatic cancer, one of the deadliest of all cancers, and to continue to develop new applications for our novel drug delivery approach.”
“We at the UNC Lineberger Comprehensive Cancer Center are thrilled to see our research endeavors, initially funded by the Unversity Cancer Research Fund, move into the clinic,” said Shelley Earp, MD, director of UNC Lineberger. “Through the cancer center’s collaborative ethos, Joe’s engineering prowess found a terrific clinical partner in Jen Jen Yeh, a pancreatic cancer surgeon with a passion to translate ideas from her lab to the clinic. Together, they did the hard but necessary work to test, develop and publish their promising findings in top notch journals. The results impressed all and with this new investment, it has the potential to help so many people in our state and beyond with hard to treat cancers.”
In conjunction with the financing, company CEO, Tony Voiers, and company founders, DeSimone and Yeh, will continue to serve on the Board of Directors.