AiM Medical Robotics Inc. (AiM), developer of MRI-compatible intraoperative robotics for neurosurgery and other applications, today announced a collaboration with Brigham and Women’s Hospital (BWH) and the Surgical Navigation and Robotics (SNR) Lab at Harvard to validate their cutting-edge robot for deep brain stimulation in Parkinson’s patients. The study will take place in BWH’s Advanced Multimodality Image-Guided Operating (AMIGO) Suite, a state-of-the-art clinical research facility. AiM also reported a successful cadaver trial at the PracticePoint facility, demonstrating precise delivery of bilateral DBS leads using real-time MRI guidance. Prior to AiM’s approach, DBS leads may miss the intended target area due to the brain shifting from the time of pre-operative imaging and surgery due to creating access holes in the skull. This demonstration highlights the robot’s ability to account for brain shift. Additionally, AiM announced a partnership with Synaptive Medical to integrate their Modus Nav neuro-navigation software with the robot, creating an optimized workflow for unparalleled precision and efficiency in neurosurgery.
AiM has partnered with the Surgical Navigation and Robotics (SNR) Lab at Harvard and entered into a clinical research agreement with the Clinical Trial Office at Brigham & Women’s Hospital (BWH). This collaboration builds upon the long-standing and successful joint research efforts of AiM’s CEO, Gregory Fischer, Ph.D., and Pedro Moreira, Ph.D. and Noby Hata, Ph.D. of the SNR Lab, with Dr. Moreira as the lead investigator for the trial at BWH. The team’s previous work includes a successful 30-patient trial for MRI-guided, robot-assisted prostate cancer biopsy. In this new endeavor, they plan to utilize AiM’s stereotactic neurosurgery robot to accurately deliver deep brain stimulation leads with real-time MRI guidance for patients with Parkinson’s Disease. AiM is thrilled to be collaborating with Dr. Reese Cosgrove, Head of Epilepsy and Functional Neurosurgery, on this groundbreaking project.
AiM Medical Robotics is bringing to market a compact, MRI-compatible surgical robot that facilitates intelligent intraoperative surgical planning and guidance through real-time soft tissue imaging. This innovative technology is crucial for tackling the issue of brain shift, which often leads to inconsistent and suboptimal outcomes in many procedures, as the target moves relative to the skull during surgery. It has been reported that 34% of deep brain stimulation lead placements require another surgery for removal or revision, largely due to missing the intended anatomical targets in the brain. AiM’s pioneering product for image-guided stereotactic neurosurgery is the result of approximately 15 years and $15M of NIH-funded academic research. The company is poised to introduce its approach to the $4.3B market, addressing challenges related to the efficient, accurate, and safe intracranial placement of neuromodulation, ablation, and drug delivery devices. By making intraoperative MRI guidance mainstream and routine, AiM aims to improve hospital throughput, procedural consistency, and patient outcomes.
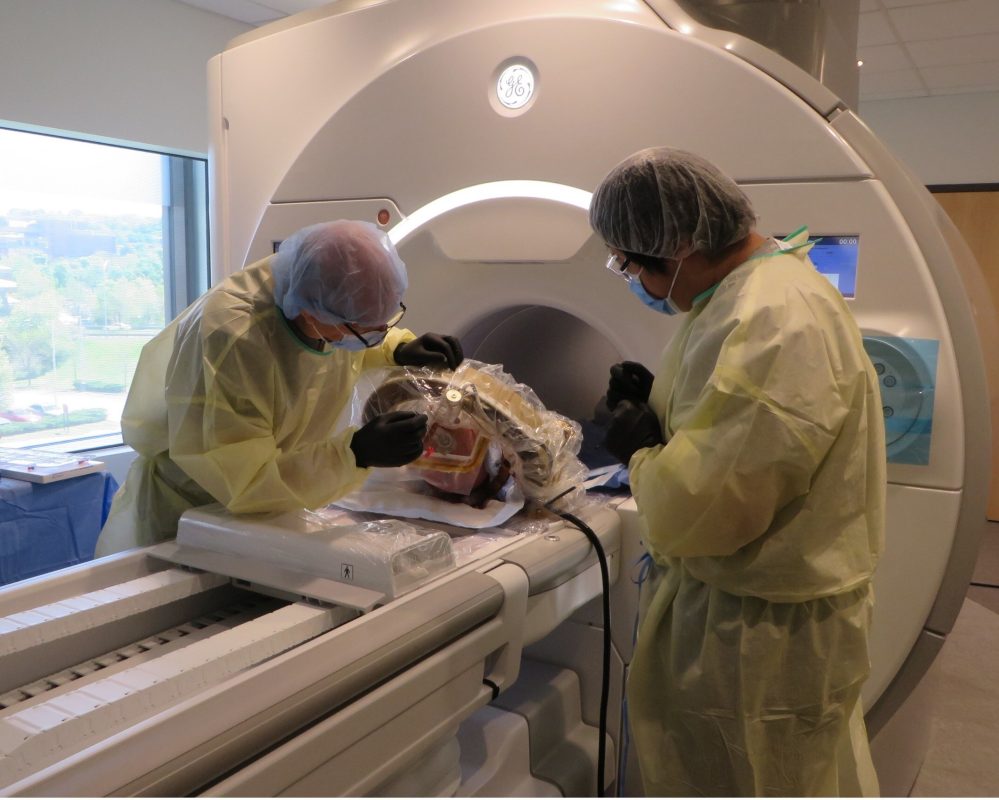
Today, AiM announced that on May 10, 2024, the company conducted a cadaver trial in collaboration with the clinical team from Brigham and Women’s Hospital (BWH) at the PracticePoint Medtech Accelerator facility located at Worcester Polytechnic Institute (WPI), where AiM is headquartered. During the trial, the team showcased AiM’s robot in a fully direct MRI-guided procedure for bilateral deep brain stimulation lead placement in a human cadaver, with the surgery performed by neurosurgeon collaborator, Dr. Cosgrove. The AiM platform demonstrated success in completing a full procedure entirely within the MRI suite, achieving high levels of efficiency and accuracy. Notably, the trial highlighted the system’s ability to identify and account for intraoperative brain shift, a phenomenon that frequently occurs during these surgeries and can impact outcomes.
AiM Medical Robotics also announced its collaboration with Synaptive Medical, Inc. (“Synaptive”), a Toronto-based Medtech company that provides solutions for surgical, imaging, and data challenges. Through this partnership, AiM and Synaptive have integrated Synaptive’s highly capable Modus Nav neuro-navigation software platform with AiM’s stereotactic neurosurgery robot, enabling enhanced visualization, navigation, and control. The platform utilizes intraoperative MRI updates to ensure precise targeting and MRI-based localization and tracking of AiM’s robot. By combining AiM’s MRI-compatible robotic stereotactic frame, Synaptive’s advanced navigation software, and intraoperative MRI, the two companies have created an optimized workflow that delivers unparalleled precision and efficiency for deep brain interventions. This collaboration marks a significant step forward in improving patient outcomes and advancing the field of neurosurgery.