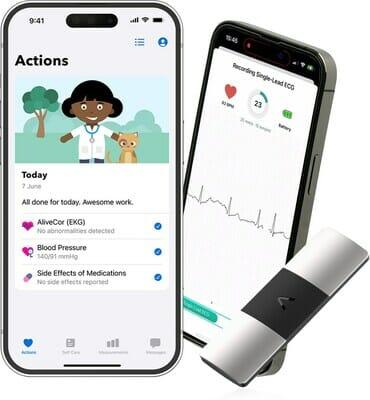
AliveCor, the global leader in personal electrocardiogram (ECG) technology, has announced a groundbreaking collaboration with Luscii, Europe’s market leader in remote patient monitoring and virtual ward provider. Together, the companies will launch the world’s first ‘virtual heart clinic in a box’, revolutionising cardiac care for millions of patients by making it easy for any hospital or GP to deliver high-quality, remote patient monitoring to their patients.
Luscii Virtual Heart Clinic® aims to provide cardiologists with a comprehensive, easy-to-use app to offer remote cardiac patient monitoring to their patients. The app prompts patients to take measurements of vital signs which are automatically analysed to detect abnormalities. This results in an improved patient experience and more time to care for the clinical teams. Luscii therefore integrates AliveCor’s advanced 6-lead ECG technology next to Omron blood pressure meters and scale and Happitech PPG in its medically certified telehealth platform that is launched in 11 countries for over 100 conditions.
“We are excited to partner with Luscii utilising AliveCor’s SDK solution, bringing the possibilities of AliveCor to Luscii’s extensive network of renowned hospitals and GP’s in Europe and Africa”, said Chris Shelford, international marketing director at AliveCor. “Luscii’s Virtual Heart Clinic, in partnership with AliveCor, will further drive the transformation of cardiac care delivery, making it more accessible and convenient for patients while reducing the burden on healthcare systems.”
Luscii’s new offering will enable healthcare providers to monitor cardiac patients remotely, reducing the need for in-person visits or admissions and ensuring that patients receive the care they need. The use of the Luscii app in cardiac care already showed impressive results across Europe, such as 81% fewer unplanned admissions in heart failure patients in London (source) and 56% fewer in-person visits for AF in Amsterdam (source). This partnership will complement these possibilities by giving heart patients more control over their own health whilst staying connected with their care team.
“AliveCor’s cutting-edge ECG technology complements our widely used remote patient monitoring and virtual ward service, and we are proud to join forces” said Luscii co-founder Dr. Joris Janssen. “Now, we can help even more patients receiving care at home and support doctors and nurses to make cardiac care more accessible, efficient, and patient-centered.”
The Luscii AliveCor SDK implementation will become available to all Luscii customers immediately with the first hospitals expected to start offering Luscii Virtual Heart Clinic with AliveCor to their patients this summer in the Netherlands, Ireland and in the UK.