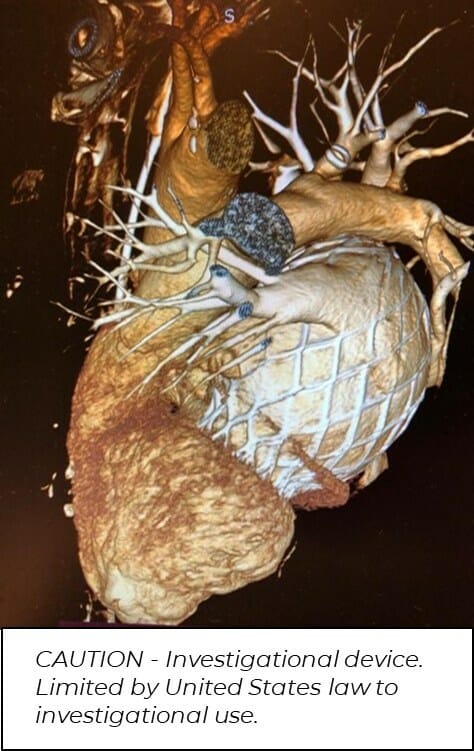
AltaValve: 4C Medical Technologies, Inc. (“4C Medical” or the “Company”), a privately-held medical technology company focused on the development of minimally invasive therapies for structural heart disease, presented its global clinical experience from its initial AltaValve Early Feasibility Study (EFS) cases at the American Association for Thoracic Surgery (AATS) 2022 Mitral Conclave Workshop on May 13, 2022 in Boston, MA.
AltaValve is a transcatheter mitral valve replacement (TMVR) device designed to broaden the treatable patient population. AltaValve’s supra-annular valve placement is intended to reduce the rate of anatomical rejections in TMVR.
Results from the AltaValve EFS show a 100% technical success rate from the first 10 patients treated via transapical (TA) approach globally. In all cases, mitral regurgitation (MR) was reduced from severe to none/trace and sustained throughout long-term follow-up.
“Based on these initial clinical experiences, the AltaValve TA procedure appears straightforward and has achieved a clinically significant reduction in MR. Patients with small left ventricular outflow tracts (LVOTs), mitral annulus calcification (MAC), and prior aortic implants were included in the study,” said Dr. Krzysztof Wrobel, a Cardiac Surgeon at Medicover Hopsital in Warsaw, Poland, who presented his own AltaValve clinical experience at AATS 2022. “AltaValve’s long-term results are promising with no current reports of thrombosis, erosion, pacemaker implantation or other device related issues within this cohort of patients. Additional clinical data collection is ongoing, including those from the transseptal approach, to further build on these experiences.”
About 4C Medical Technologies, Inc. 4C Medical is a medical device company developing minimally invasive technologies for structural heart disease, focusing initially on mitral regurgitation (MR) therapy and subsequently on tricuspid regurgitation therapy. Subject to regulatory approvals, the Company’s AltaValveTM would be the first MR treatment with atrial-only fixation that is designed to minimize known issues associated with current transcatheter mitral valve replacement (TMVR) technologies, which rely on fixation within the native mitral annulus. The AltaValve is an investigational device only, has not been approved for marketing by the Food and Drug Administration or any other regulatory agency, and is not available for sale.