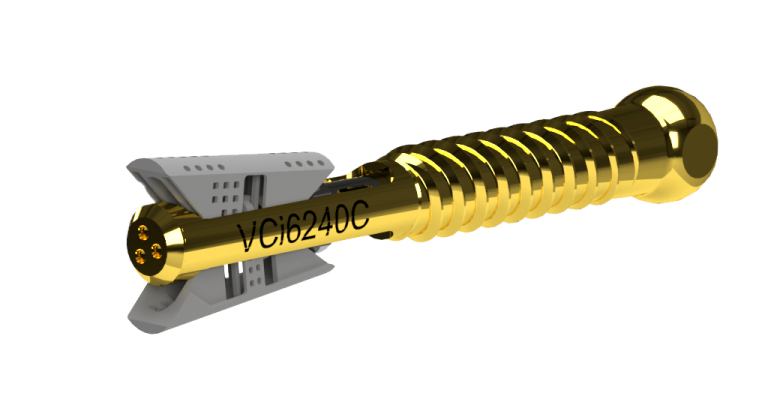
Amber Implants, an innovative medical technology company developing next-generation implants for spinal injuries, today announces the official start of the clinical trial for the VCFix® Spinal System.
This first-in-human clinical trial will assess the safety and effectiveness of the VCFix® Spinal System implant for patients suffering from vertebral compression fractures. The implant is provided with a user-friendly, single-use sterile surgical kit, ensuring perfect traceability and reducing the risk of infection.
The study is being led by Prof. Dr. Robert Pflugmacher, at the Clinic for Orthopaedics of the Mechernich Hospital in Germany. He was very pleased with the device’s performance regarding the ease of the procedures and the initial results for the patient.
Dr. Banafsheh Sajadi, Co-Founder and Chief Executive Officer, Amber Implants added “This news marks a key milestone in the product development of the VCFix® Spinal System, and we aim to expand the clinical trial to further validate the device’s versatile applications. Our comprehensive approach is a significant leap forward in spinal healthcare, promising safer and more effective treatments for patients with both traumatic and osteoporotic vertebral fractures.”
Over 8.6 million people per annum suffer from different types of vertebral fractures. These fractures cause severe back pain, limited mobility, and an increased rate of mortality, in an already vulnerable elderly population. Currently, vertebral compression fractures are managed through the injection of Polymethyl methacrylate (PMMA) bone cement into the affected vertebra or with multi-level posterior fixation. This can lead to side effects in up to two-thirds of patients, post-treatment.
The VCFix® Spinal System offers an innovative strategy for managing a diverse range of vertebral fractures and is poised to eliminate the need for bone cement while ensuring continued compatibility. This system is designed to be applicable for both single- and multi-level posterior fixation and is engineered for superior mechanical strength, maximum fracture reduction, and anatomical restoration. The VCFix implant not only encourages natural healing but also enhances stability and optimizes load distribution within the spine through pedicle anchorage.