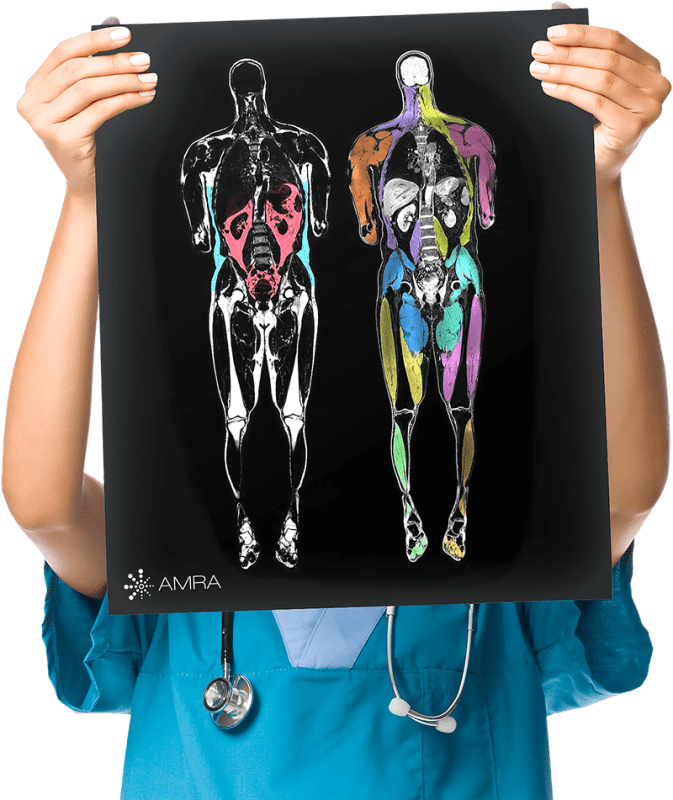AMRA Medical, a digital health company delivering a new standard in body composition analysis through rapid whole-body magnetic resonance imaging (MRI), today announced its involvement with the Facioscapulohumeral Muscular Dystrophy (FSHD) Clinical Trial Research Network (CTRN) to support the Motor Outcomes to Validate Evaluations Plus (MOVE+) Study—a natural history study for people living with FSHD.
MOVE+ is a sub-study of the ongoing natural history study called Motor Outcomes to Validate Evaluations in FSHD (MOVE FSHD) and adds MRI—made possible in part through sponsorship by Avidity Biosciences, FSHD Society, Friends of FSH Research, and FSHD Canada. MOVE+ will enroll 200 participants across 12 sites in the US and two planned sites in Canada with the goal of better understanding how to utilize whole-body MRI to discover and validate specific biomarkers for FSHD that can inform future clinical trials. The results of this study will be particularly important for MOVE+ sponsoring companies like Avidity Biosciences—a biopharmaceutical company developing a new class of RNA therapeutics with an advancing and expanding pipeline that includes multiple skeletal muscle programs.
“The FSHD CTRN is very excited about our collaboration with AMRA. We think that the use of whole-body MRI will be important not only for trial planning, but to better understand and treat people with FSHD,” says Jeffrey Statland, MD, Professor of Neurology at the University of Kansas Medical Center, which also is the Clinical Coordinating Center for the FSHD CTRN.
To date, the MOVE study has used functional tests as primary outcomes, but recent studies have shown the promise of MRI for FSHD. To complement functional tests, MOVE+ will use AMRA’s whole-body, whole-muscle MRI protocol and analysis, which involves measuring 36 muscles throughout the participant’s shoulders, arms, torso and legs—distal to proximal and bilaterally. Specifically, the researchers will quantify muscle fat infiltration, lean muscle volume, and muscle fat fraction at two–time points throughout a three-year study period for each individual.
Evaluating entire muscles throughout the whole body provides a fuller picture of how the disease presents and progresses, which is critical to understand when searching for the most optimal outcome measurements to use in FSHD clinical trials. Not only is this applicable for FSHD, but also other neuromuscular disorders including DMD, LGMD, Myotonic Dystrophy, Mitochondrial Myopathy, SBMA, SIBM, and Pompe Disease.
Other past news.