Contributing to this year’s theme at the annual EWMA Conference, ‘Collaborative and Sustainable Wound Care’, experts from Avery Dennison Medical and Polaroid Therapeutics will lead an insightful discussion. Discover how partnership can bring a unique approach to antimicrobial wound dressings.
Polaroid Therapeutics
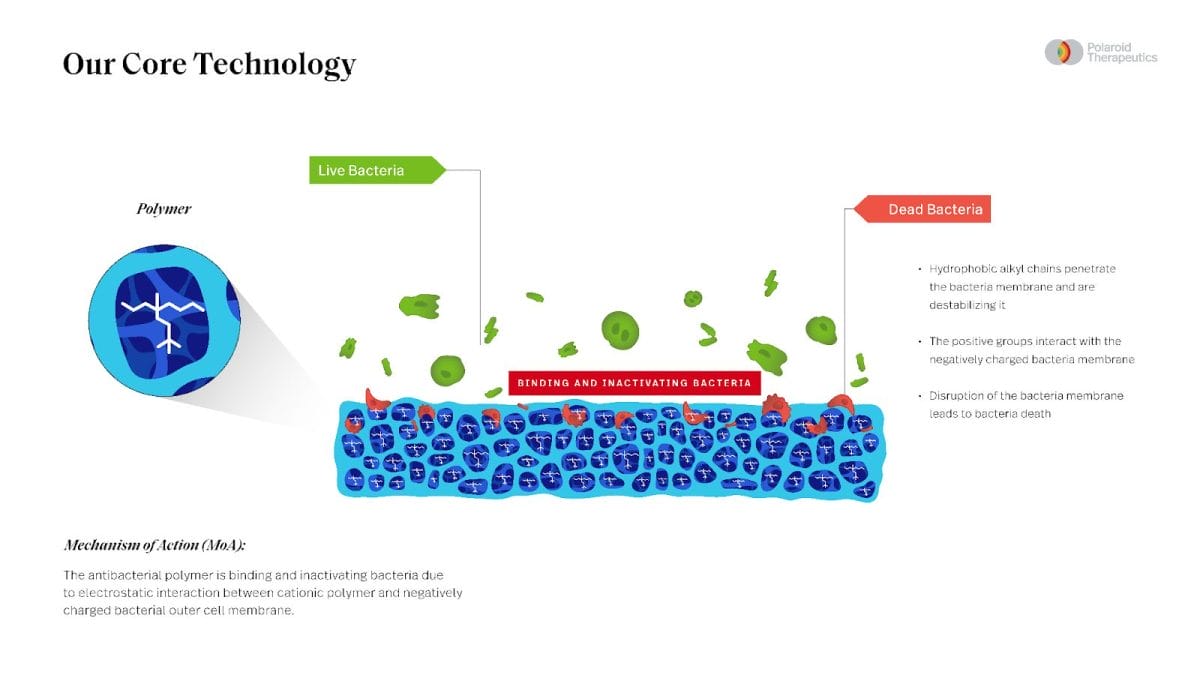
The 20-minute session explores the synergy of Polaroid Therapeutics’ cutting-edge technology in the development of antimicrobial compounds and Avery Dennison Medical’s renowned expertise in materials science and wound care solutions, resulting in the creation of an innovative antimicrobial platform that elevates patient care and provides solutions to modern global health challenges.
Details of the session:
Title: Polaroid Therapeutics & Avery Dennison Medical: How the power of partnership brings a novel approach to antimicrobial wound dressings.
Speakers: Mr Ran Frenkel, CEO, Polaroid Therapeutics; and Dr. Emmett McArdle, Advanced Research and Development Manager at Avery Dennison Medical.
Date and time: Wednesday, May 1st, 2024, from 15:15 to 15:35.
Room: EWMA ARENA (E-Poster Area).
Conference venue: ExCeL London.
All participants to the EWMA Conference can attend this session, no prior registration is needed.