Append Medical
Append Medical, developer of the Appligator™, a novel implant-free left atrial appendage (LAA) exclusion device, today announced completion of its first ever pre-clinical chronic procedure.
Append Medical notes the procedures were completed with IMMR, one of the world’s leading preclinical laboratories and paves the way for the first human trial.
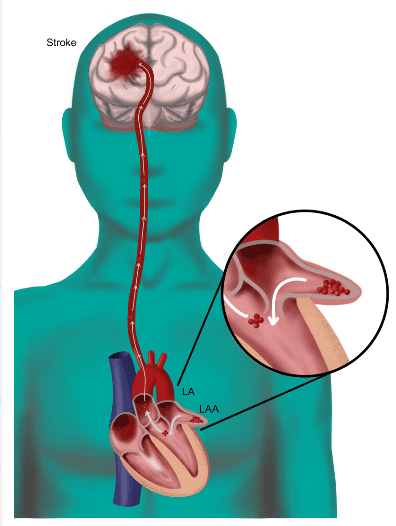
Append’s team was granted special permission to enter France by its Ministry of the Interior based on the promise its technology holds for stroke prevention in atrial fibrillation patients, a major global health concern.
“These first procedures provide an important validation for our approach, which is intended to significantly simplify the goal of sealing the LAA and preventing stroke in AF patients without leaving any implant in the heart,” said Zachi Berger, PhD, Founder and CEO of Append Medical. “Organizing this trial during the current pandemic was not a simple task and we would like to thank the relevant French bodies as well as the IMMR team for allowing us to complete this trial while complying with COVID-19 restrictions.”
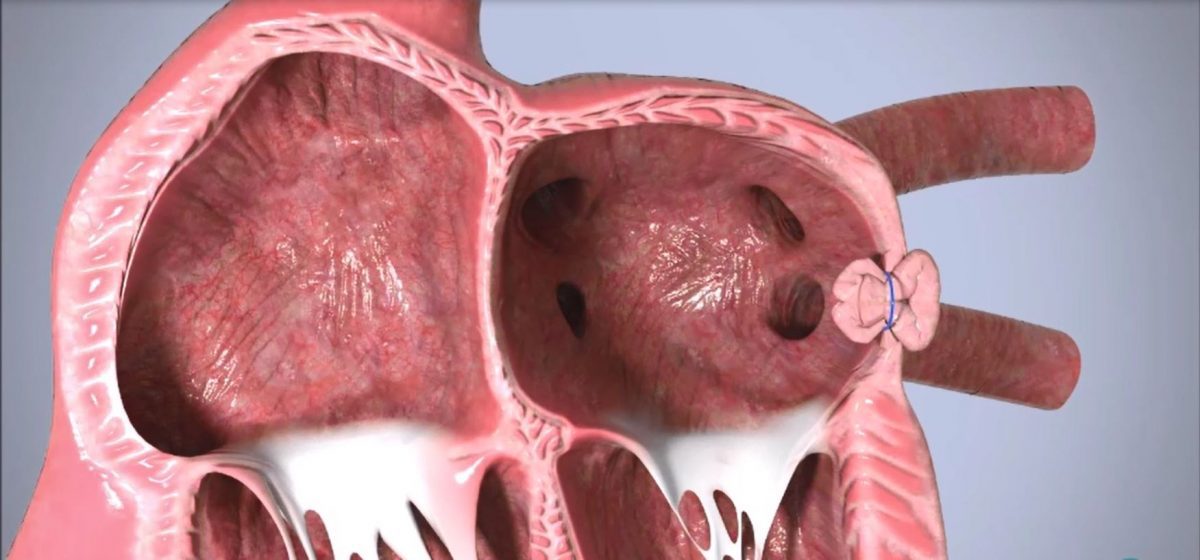
“My team and I found the procedure to be elegant and straightforward toward continuing to make LAA exclusion more accessible,” said Dr. Nicolas Borenstein, DVM, PhD. Scientific and Technical Director at IMMR. Both animals are doing fine so far, and the post-procedure images are spectacular. Congratulations to the Append team on this achievement.”
The Append Medical “Appligator™” is designed to reduce stroke risk in AF patients by excluding the LAA to prevent blood clot leakage with a minimally invasive transseptal intervention leaving no implant behind. The Appligator device utilizes natural tissue manipulation to achieve complete LAA closure through invagination of the LAA into
the left atrium. This approach is designed to minimize device-related thromboembolism risk by leaving only a suture – and no metal implant – at the closure site.
Append Medical operates as part of MEDX Xelerator in Israel. MEDX Xelerator’s partners include MEDX Ventures, Boston Scientific Inc., Intellectual Ventures and Sheba Medical Center.