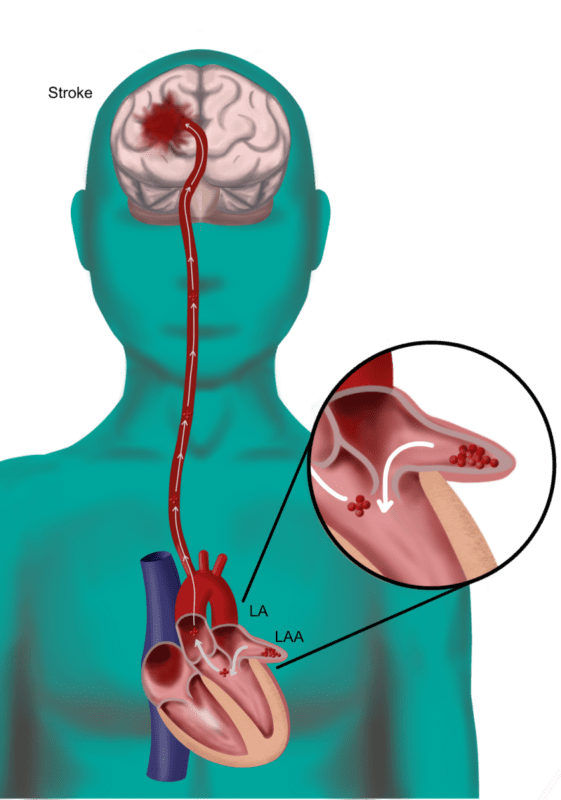
Append Medical, developer of the first no metal implant minimally invasive solution for transcatheter Left Atrial Appendage (LAA) exclusion, today announced the completion of a $7.4 million Series A financing round.
The round will enable the company to complete development and perform its first clinical trial.
Originally developed based on the research of Prof. Leonid Sternik from the Sheba Medical Center, the company was founded and led by Dr. Zachi Berger with seed investment from the MEDX Xelerator, a leading MedTech incubator in Israel.
“Append Medical brings an important innovation to the field of stroke prevention in atrial fibrillation patients,” explains Prof. Horst Sievert. “Its suture delivery system is the first ever to allow for minimally invasive endovascular exclusion of the left atrial appendage leaving only a suture lasso behind and no metal implant.”
The financing round was led by MedTecX LP along with an unnamed strategic company and other investors and included a $700K of matching grant from the Israel Innovation Authority.
“This round will enable Append to continue developing its technology towards a clinical product. We are thrilled to see the accomplishment of the team so far,” noted Shai Policker, CEO of the MEDX Xelerator and Append’s Chairman.
“The growing problem of atrial fibrillation and the issues associated with anticoagulants calls for innovation and we are happy to take part in supporting this important technology,” added Lifei Cheng, CEO of MedTecX.