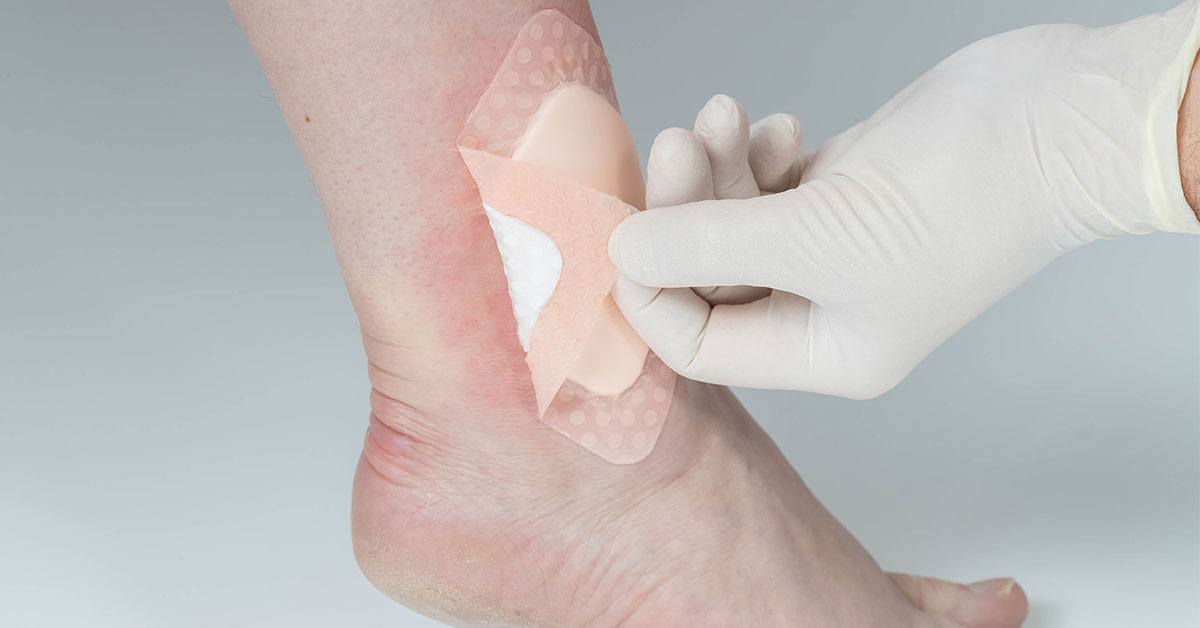
Avery Dennison Medical, a leader in adhesives for medical applications, announces the launch of its upgraded SilFoam Lite, featuring MDR certification, improved performance and sustainability advancements.
The newly enhanced SilFoam Lite delivers superior efficiency and reliability, bringing improved fluid handling capabilities and improved tack. These improvements make the product ideal for customers seeking quality, high-performance solutions in wound care.
Committed to sustainability, Avery Dennison Medical has also made significant strides in sustainability with this product. The latest SilFoam Lite incorporates a thinner 100gsm adhesive layer, reducing the amount of silicone used to achieve an even better performance.
In addition to its performance and environmental benefits, the new SilFoam Lite is now MDR certified, allowing customers to use this certification in their end products. The certification process proves the high quality standards of the product and Avery Dennison Medical.
“Our goal is to consistently improve our products, and the MDR certification shows our dedication to quality products,” said Paul Saunders, Senior Global Marketing Manager at Avery Dennison Medical. “The improved SilFoam Lite combines improved performance and sustainability, making it a compelling choice for today’s medical professionals.”