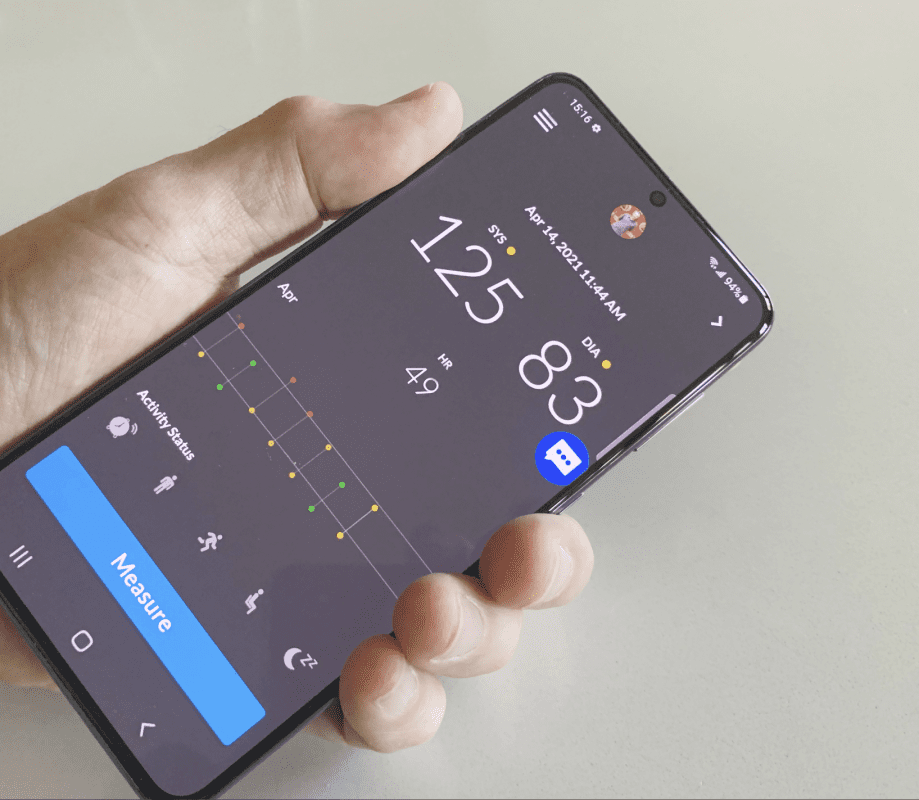
Biospectal SA, the remote patient monitoring and biosensing software company, today announced the closing of a $4.3M (€3.7M Euros) round of seed funding led by digital health investor, SeedLink, with additional funding from LabCorp, Athensmed, Swiss based Privilege Ventures and other European and US investors. Biospectal will leverage the new funds to scale its OptiBP™ smartphone blood pressure monitoring application and data platform technology worldwide.
Biospectal OptiBP for Android launched in public beta in January 2021. Biospectal OptiBP for iOS is currently in alpha and is planned for public beta launch in the second half of 2021. View a short video of how Biospectal OptiBP works here.
“Biospectal’s technology has the ability to transform the global network of smartphones into a connected, clinical-grade blood pressure monitoring platform that shifts clinical capabilities out of the traditional institutional settings,” said Biospectal CEO and co-founder, Eliott Jones. “The funding we’ve received will accelerate Biospectal’s growth trajectory along with the appointments of Silicon Valley-based, interventional cardiologist and influential serial healthcare innovator, Dr. Fred St. Goar, MD, Tim Juergens, General Manager and co-founder of SeedLink, William Hanlon from LabCorp and Kustaa Piha, successful Health Tech Entrepreneur and Investor from Athensmed to the Biospectal Board of Directors,” added Reinhard Stary, Executive Chairman of the Board.
How Biospectal OptiBP Works
Biospectal’s OptiBP medical-grade app uses a smartphone camera’s optical lens to easily measure and record a user’s blood pressure flow via their fingertip. Biospectal’s proprietary algorithms and optical signal capture methods transform the captured data into blood pressure values in approximately 20 seconds — half the time of a typical blood pressure cuff. With Biospectal OptiBP anyone in the world with a smartphone can turn their device into a connected, smart, clinical-grade monitor in the time it takes to download and install an app. OptiBP’s data also connects seamlessly with clinicians to support treatment regimens that help improve health and quality of life. Biospectal’s patented OptiBP technology replaces the antiquated, impractical traditional blood pressure cuff and brings medical-grade blood pressure measurement to the ‘point of patient’ without the need for yet another wearable device or additional bulky hardware that can break or needs to be replaced.
According to the CDC, hypertension affects nearly 46% (100M) of US adults, and the World Health Organization estimates 1.13Bn people worldwide suffer from the disease. Dubbed the “silent killer,” only one in five people afflicted with hypertension has it under control.
“I’m honored to join Biospectal’s Board of Directors. Addressing the global hypertension crisis through more frequent and meaningful monitoring has become more critical and important than ever — a need that the onset of COVID-19 and demand for telehealth solutions has only amplified. People need an easy to use, digital means to accurately measure, monitor, track and share their blood pressure data with their doctors. Biospectal is the first company I’ve encountered in the remote patient monitoring sector to bring an accessible, secure, and customer-centric blood pressure monitoring technology to market at global scale,” said Dr. Fred St. Goar, MD. “Biospectal OptiBP is a game changer that promises to have a huge impact on improved access to and treatment of hypertension management.”
Founded in 2017 and supported by grants from the Bill & Melinda Gates Foundation, Grand Challenges Canada and Innosuisse, Biospectal’s patented technology was developed and validated in partnership with the Swiss Center for Electronics and Microtechnology (CSEM) and represents 10+ years of non-invasive optical biosensing R&D. A recent large-scale, third-party clinical research study published by Scientific Reports in Nature validated the OptiBP app’s ability to measure blood pressure as accurately as a standard cuff. Additionally in May 2021, Biospectal announced two independent global research and validation studies funded by the Bill & Melinda Gates Foundation and Global Grand Challenges Canada’s Saving Lives at Birth initiative. These studies further Biospectal’s ability to transform the global network of smartphones into a connected, clinical-grade blood pressure monitoring platform — democratizing access and bringing the power of remote patient monitoring to people and communities worldwide.
“Biospectal OptiBP is a breakthrough technology that enables more accessible, effective, and efficient patient monitoring to empower patients to contribute to, and participate in, the clinical regimen for chronic condition monitoring and management,” said Tim Juergens, General Manager, and co-founder, SeedLink. “The ‘participatory patient’ dynamic is accelerating and forms an integral component within the research and clinical landscape. Biospectal answers the increasing demand for telehealth solutions and will help improve the health and quality of life for millions and we are excited to support Biospectal’s future success.”