Biotium, a leading provider of innovative life science solutions, is thrilled to announce the release of its latest product, Mix-n-Stain™ CF® Dye IgM Antibody Labeling Kits. These groundbreaking kits revolutionize the labeling process for IgM antibodies, enabling researchers to easily obtain high-performance fluorescent IgM conjugates with minimal hands-on time with no purification after labeling.
IgM antibodies offer advantages over IgG antibodies due to their increased avidity. However, traditional methods for conjugation of IgG antibodies are frequently ineffective for optimal labeling of IgM antibodies due to differences in the immunoglobulin structures. Recognizing this roadblock, Biotium has successfully optimized the reaction conditions and developed Mix-n-Stain™ CF® Dye IgM Antibody Labeling Kits to address this issue.
With these kits, researchers can now label 25 ug or 100 ug of their IgM antibody in as little as 15-30 minutes, ensuring efficient and rapid experimental workflows. These kits offer unparalleled convenience, with less than 30 seconds of hands-on time required, saving valuable laboratory resources.
Mix-n-Stain™ CF® Dye IgM Antibody Labeling Kits are available in a selection of 8 CF® Dyes and FITC. CF® Dyes are Biotium’s next-generation fluorescent dyes designed to provide superior brightness and photostability compared to other commercially available fluorescent dyes.
Features and benefits of the Mix-n-Stain™ CF® Dye IgM Antibody Labeling Kits
- Optimized for IgM: Developed to offer high-performance IgM conjugates
- Quick and easy: Conjugate in as little as 15-30 minutes, no purification required
- Wide selection: Available in your choice of 8 bright CF® Dye colors and FITC
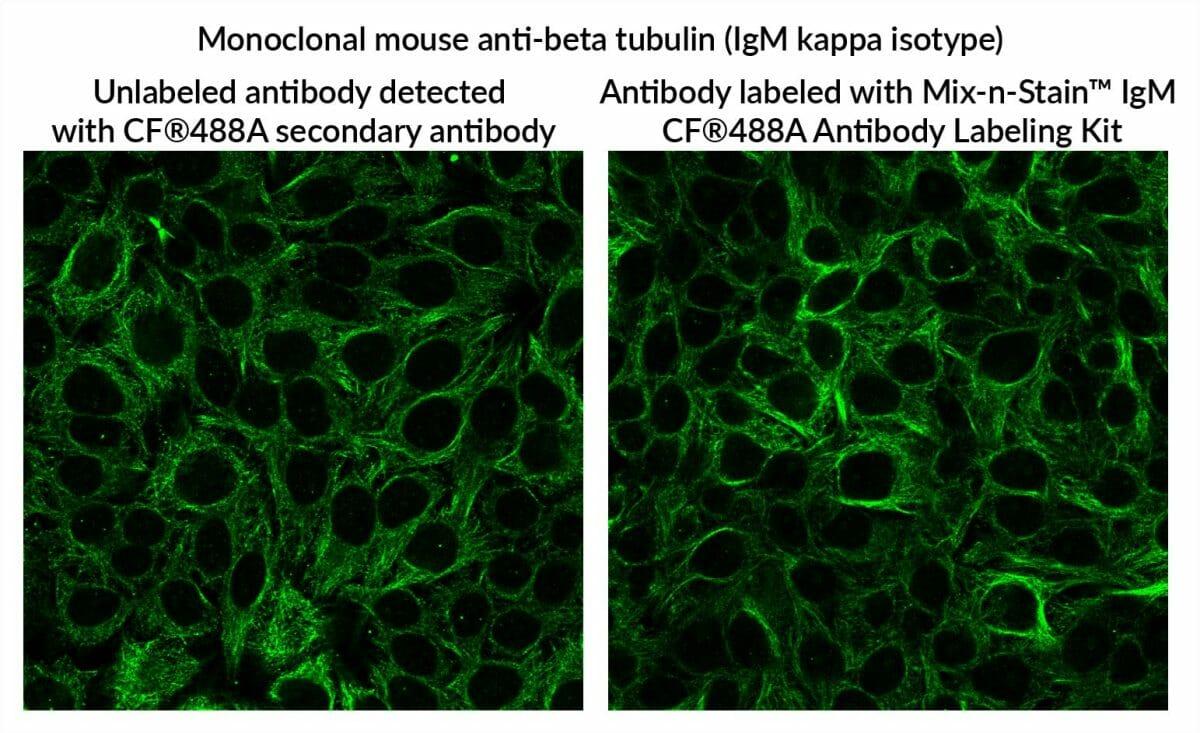
Mouse anti-beta tubulin clone 5H1 (IgM kappa) was labeled with CF®488A using Mix-n-Stain™ IgM labeling, and then was used to stain methanol-fixed HeLa cells at 5 ug/mL (right panel). The conjugate shows comparable staining specificity compared to unlabeled antibody at 1 ug/mL detected using CF®488A Goat Anti-Mouse IgM Mu chain secondary antibody at 2 ug/mL (left panel).
“We are excited to introduce the Mix-n-Stain™ CF® Dye IgM Antibody Labeling Kits to the scientific community,” says Dr. Lori Roberts, Director of Bioscience at Biotium. “This technology provides a new and simple solution to the longstanding challenge of IgM conjugation. We believe these kits will provide researchers with more flexibility in panel design for immunofluorescence and flow cytometry.”
For more information about the Mix-n-Stain™ CF® Dye IgM Antibody Labeling Kits, please visit the product page.
Biotium also offers a range of other labeling solutions to meet diverse research needs. The Mix-n-Stain™ CF® Dye Antibody Labeling Kits are ideal for rapid and efficient labeling of IgG antibodies, while the Mix-n-Stain™ Nanobody Labeling Kits cater to the labeling of single-domain Nanobodies®. Biotium also provides Mix-n-Stain™ kits for labeling antibodies with biotin, DNP, or DIG. Browse Biotium’s full catalog of Mix-n-stain™ Antibody Labeling Kits.