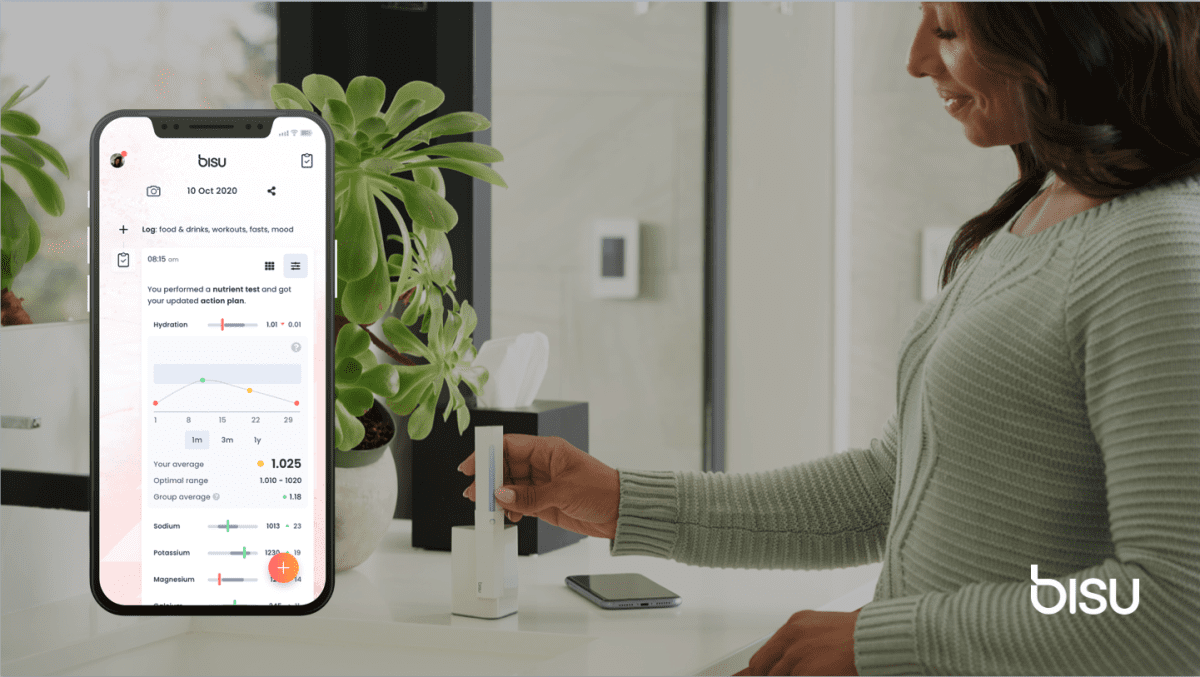
Bisu, Inc., a startup that helps people improve their health and wellness through lab-grade testing at home, today announced a new round of seed funding of $3.2 million. Leading the round was Korean biotech investor QUAD, with participation from ASICS Ventures Corporation, 15th Rock Ventures, Pacifico Investments, and SOSV, the world’s most active investor in hardware and health tech investing for the third time. The funding will enable Bisu to bring to market Bisu Body Coach, a portable “home health lab” for personalized nutrition and lifestyle advice through easy, accurate urine and saliva testing. Bisu also reached an agreement with ASICS Corporation to collaborate on health and fitness service initiatives.
Bisu Body Coach consists of a disposable test stick and a reader that syncs with a companion smartphone app. The microfluidic “lab-on-a-chip” technology used in the test stick enables reliable, precise and automated measurements of a wide range of biomarkers in just two minutes.
Bisu Body Coach app provides feedback on key nutrition indicators such as hydration, minerals and vitamins, and recommendations are personalized based on the user’s goals, dietary preferences, activity, sleep and weight. Bisu Body Coach is currently in beta, with a pipeline of additional test sticks covering training intensity, hormones, and pet and baby health. Bisu and ASICS will work together on future initiatives aimed to enhance personal health and athletic performance. ASICS’ investment follows Bisu’s victory in the U.S. Sports & Fitness Industry Association’s startup competition — one of the industry’s major events for which more than 100 sports and fitness executives served as judges.
“We decided to invest in Bisu because we believe its home health lab technology, which provides affordable access to actionable health data, is very innovative and has huge potential,” says Hiroaki Kageyama, President of ASICS Ventures. “We are thrilled to build this collaboration with Bisu as a strategic partner”.
“We’ve been flushing away insight and data for centuries,” says Duncan Turner, SOSV General Partner and HAX Managing Director. “Bisu is showing how valuable capturing and acting on that data can be for human health”.
“This funding is a major vote of confidence from a group of investors who are both tech-savvy, consumer-focused and globally minded,” says Daniel Maggs, Co-founder & CEO at Bisu. “We are delighted to announce our collaboration with ASICS. ASICS shares our passion for making beautiful yet scientifically rigorous products in the spirit of Japanese craftsmanship, yet is also a modern, globally-recognized brand,” says Wojciech Bula, PhD, Co-founder & CTO.