September 1, 2020
3 UnityPoint Health hospitals in Illinois experienced smoother workflow, an uptick in efficiency and excellent image quality with Carestream Health’s DRX-Evolution Plus and DRX-Revolution diagnostic imaging systems. The news was announced today by Carestream Health, a leader in this field.
“The workflow is more efficient, it’s faster with these new systems. We see a more productive use of time, which means faster turnaround for both our clinicians and our patients,” said Kevin Baker, Regional Director of Medical Imaging for UnityPoint Health’s Methodist, Pekin and Proctor hospitals. “There are different ways to use the equipment that we didn’t have before with other units.”
Carestream’s X-Factor platform enables facilities to share the same wireless detector seamlessly across all Carestream DRX products and allows technologists to move easily between different systems.
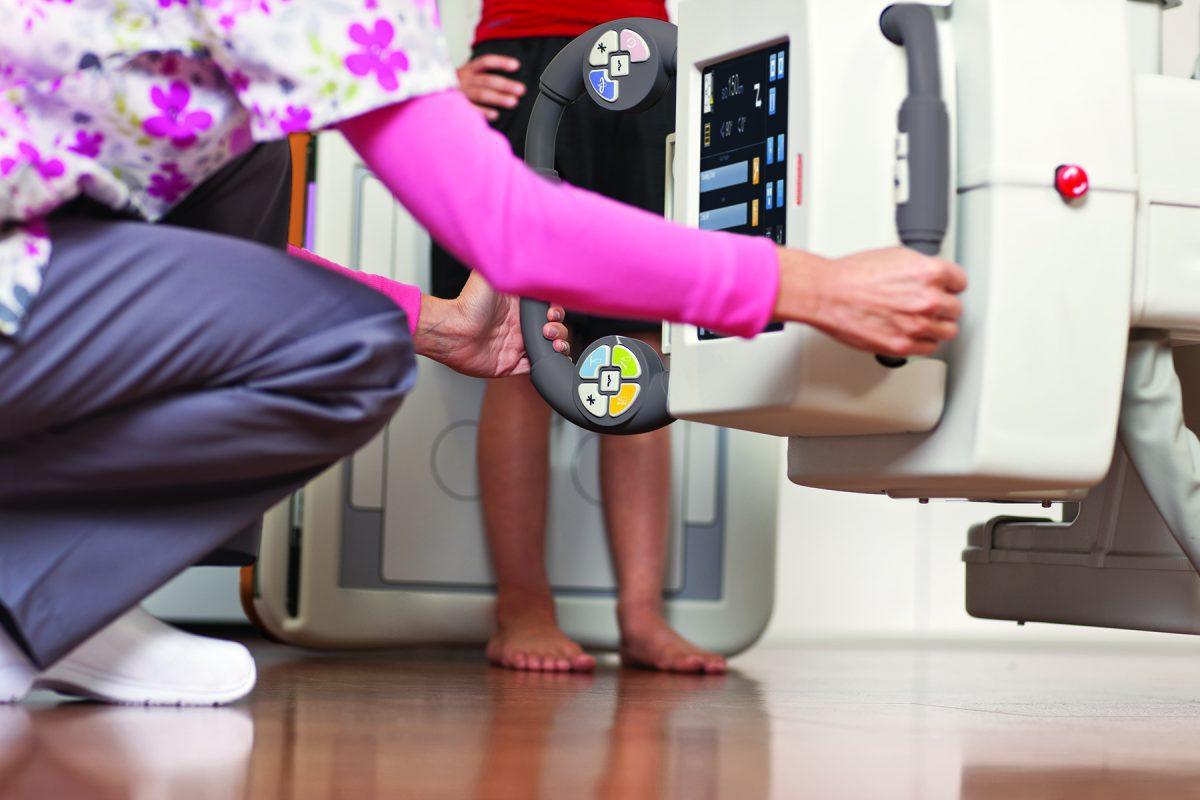
With the CARESTREAM DRX-Evolution Plus System, UnityPoint’s emergency departments perform a variety of general radiographic exams with ease. Its user-friendly software along with the ability to operate the system in various positions for examination empower hospitals to expand their imaging solutions and enhance productivity. The DRX-Evolution Plus (see video link) enables technologists to capture a wide array of X-ray images, including cross-table, spine, abdomen, long-length and scoliosis exams.
“The digital image quality is above and beyond,” Mr. Baker said. “We are able to tweak and substitute protocols if needed. Service is prompt and we’ve been very happy with that.”
On the portable imaging front, the CARESTREAM DRX-Revolution Mobile X-ray System is used throughout UnityPoint’s hospitals as needed, from patient rooms and the Intensive Care Unit to operating rooms. Designed to quietly navigate hallways and work in tight spaces, the DRX-Revolution provides high-quality image capture at the patient’s bedside.
“The mobile X-ray system is our primary portable unit for all exams. The image quality is preferred over other units by our radiologists,” Mr. Baker said.
With these new medical imaging systems, having the ability to work with a lower radiation dose and still achieve high-quality digital images has been especially useful at UnityPoint. In addition, quick access to technical support makes it easy for the hospitals to work without significant interruption in workflow.
“We want to use as little radiation as possible and get good image quality,” Mr. Baker said. “Carestream’s ability to work with us and make the adjustments that we want is very helpful.”
Establishing a trusted relationship with Carestream—from sales to customer support—has been instrumental in the expansive and cohesive use of the digital imaging systems at UnityPoint.
“It really starts with ‘Do you trust the company? Do you trust what they tell you? Has it been proven over time?’ Building a partner-type relationship is critical,” Mr. Baker added. “They understand the challenges we face with funding and how we need to get the most with our dollar. They are selling products that we need and not pushing products that we don’t. To me, that’s invaluable.”