April 19, 2021
Carestream Health in partnership with Ziehm Imaging announced the addition of a mobile C-arm into its growing innovative product portfolio.
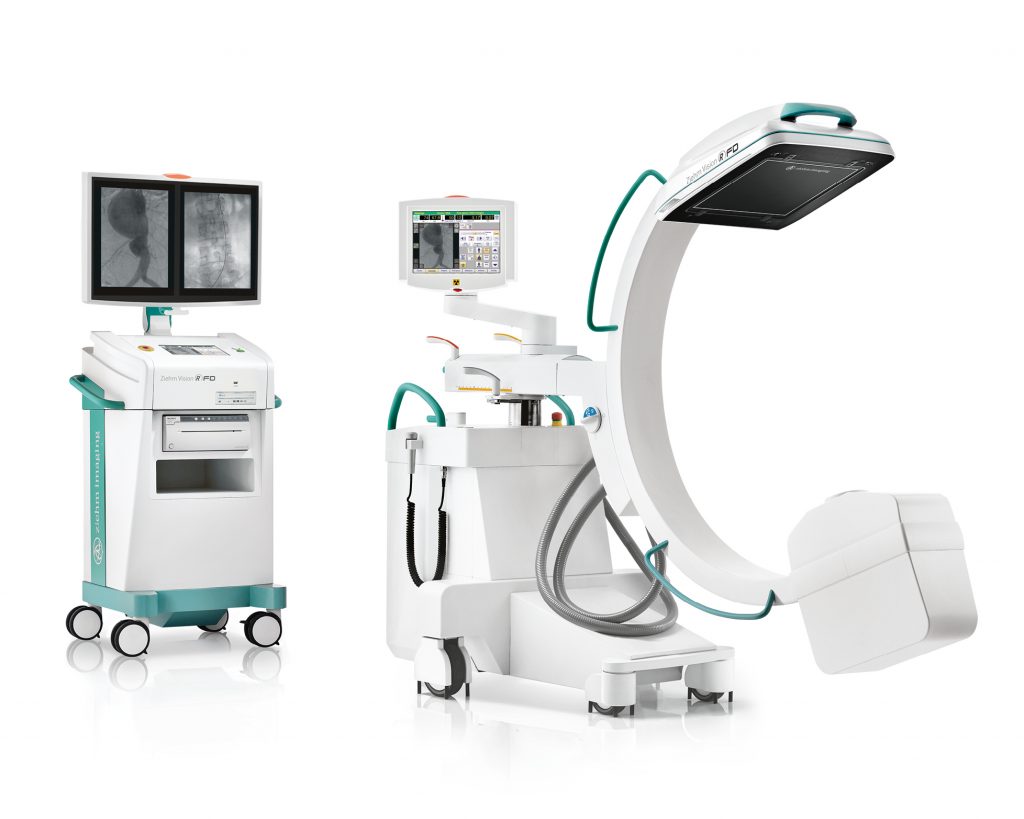
Known as the Ziehm Vision RFD C-arm, this surgical imaging system will further enhance Carestream’s mobile and fluoroscopic product offerings to benefit even more healthcare providers. The system will be available in the U.S. and Canada in 2021.
The Vision RFD C-arm offers a 25kw generator, in either a 20.5 x 20.5 cm or 31 x 31 cm field of view flat-panel CMOS digital detector, allowing for broad procedural work including vascular, cardiac, spine ortho-trauma and pain management, in addition to many general surgical applications. The system’s Advanced Active Cooling enables extended case times beyond traditional air-cooled systems. It also provides an intuitive graphical interface that benefits technologists with reduced training time as well as improved productivity.
“The partnership with Carestream Health confirms Ziehm Imaging’s industry-leading technology,” said Nelson Mendes, President and CEO of Orthoscan and Ziehm Imaging Inc. “As a result of this partnership, we’re able to provide Carestream and its customers with one of the most innovative C-arms on the market. The Ziehm Vision RFD delivers advanced surgical care with the Ziehm Usability Concept, provides exceptional image quality and helps to reduce exposure significantly with the next-generation SmartDose, thereby providing a very unique package.”
Ziehm Vision RFD systems will be available for sale and service through Carestream. The company’s Sales & Service team—working in collaboration with Ziehm Imaging—will provide responsive support to all valued customers. Carestream Service is widely recognized for its outstanding customer satisfaction and prompt support.
“The addition of the Ziehm C-arm to our medical imaging portfolio—backed by Carestream’s deep experience in radiology and fluoroscopy applications—is another example of our commitment to meet the evolving needs of our customers and their patients,” said Steve Romocki, Worldwide Product Line Manager for Radiology Systems at Carestream.