Carestream Health will showcase its innovations in mobile medical imaging with the Ziehm Vision RFD C-arm and DRX-Revolution Mobile X-ray System at the Radiological Society of North America (RSNA) conference in Chicago, Ill.
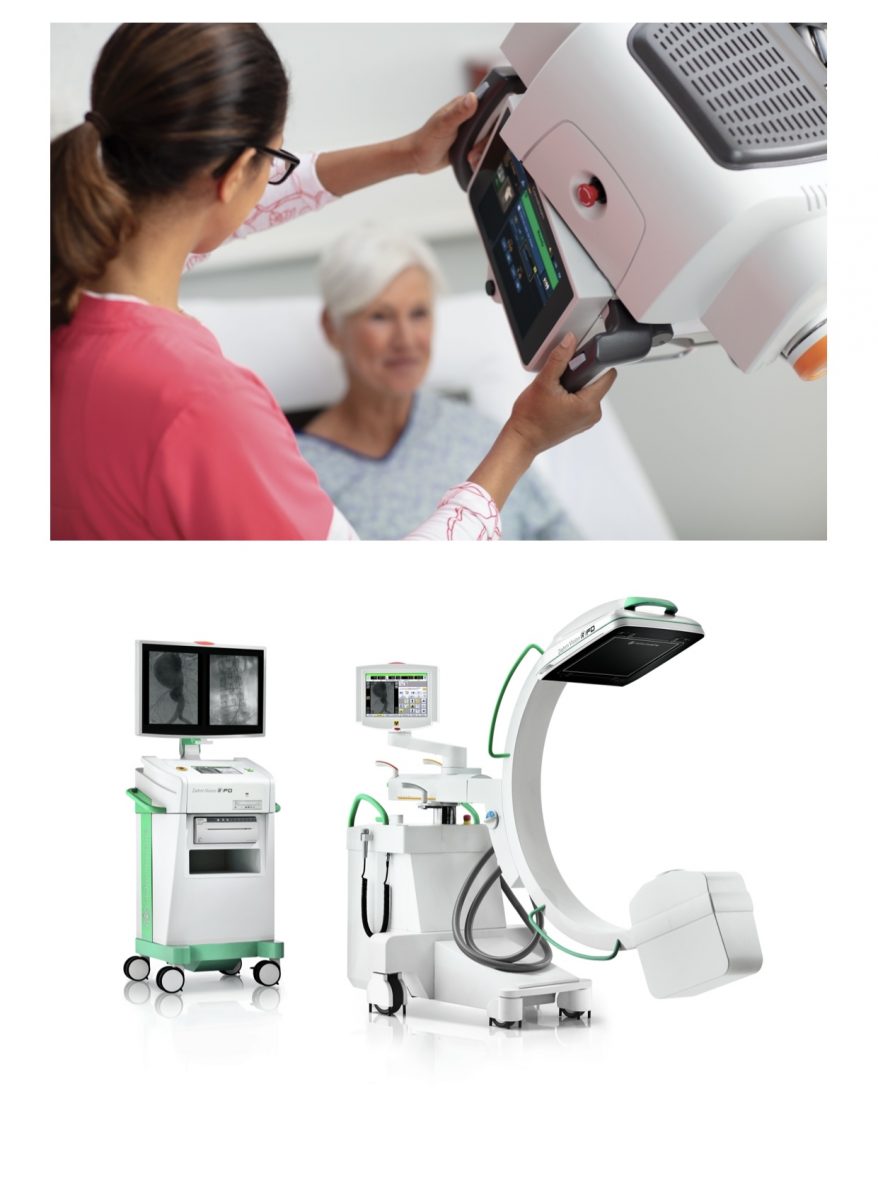 With a continued focus on advanced diagnostic imaging, Carestream recently added the Ziehm Vision RFD C-arm System to its product portfolio. A mobile, fluoroscopic surgical imaging system with a flat-panel detector, the Ziehm Vision RFD C-arm allows for broad procedural work including vascular, cardiac, spine ortho-trauma and pain management, in addition to many general surgical applications. The system’s Advanced Active Cooling enables extended case times beyond traditional air-cooled systems. It also provides an intuitive graphical interface that benefits technologists with reduced training time as well as improved productivity.Carestream offers this system in partnership with Ziehm Imaging, bolstering its mobile and fluoroscopic product offerings to further benefit healthcare providers.
With a continued focus on advanced diagnostic imaging, Carestream recently added the Ziehm Vision RFD C-arm System to its product portfolio. A mobile, fluoroscopic surgical imaging system with a flat-panel detector, the Ziehm Vision RFD C-arm allows for broad procedural work including vascular, cardiac, spine ortho-trauma and pain management, in addition to many general surgical applications. The system’s Advanced Active Cooling enables extended case times beyond traditional air-cooled systems. It also provides an intuitive graphical interface that benefits technologists with reduced training time as well as improved productivity.Carestream offers this system in partnership with Ziehm Imaging, bolstering its mobile and fluoroscopic product offerings to further benefit healthcare providers.
Carestream’s enhanced DRX-Revolution Mobile X-ray System has an improved ergonomic design and streamlineduser workflow, as well as functional LED lighting and more responsive display screens. Dedicated to both patient and provider safety, Carestream’s mobile solution plays an instrumental role in limiting the potential spread of infectiousdiseases by allowing operators to complete all general imaging examinations at the bedside.
“Carestream’s mobile and fluoroscopic product offerings deliver high-quality diagnostic images at the bedside—whether that is in the ICU, OR or anywhere else—reducingthe need to transport patients throughout a facility,” said Jorge Quant, VP Strategy and Marketing, Americas Region and Global Marketing Director at Carestream. “Both the Ziehm Vision RFD C-arm and our mobile DRX-Revolution are examples of Carestream’s commitment to meet the evolving needs of our customers and their patients.”
To learn more about Carestream’s continuous advances in mobile imaging, stop by the Carestream booth #7316 in North Hall during RSNA 2021.