Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, and Carilion Clinic, a not-for-profit health care organization serving more than one million people in the States of Virginia and West Virginia, today announced Carilion Clinic will adopt the Philips cardiovascular care ecosystem of solutions at the future home of its Cardiovascular Institute at Crystal Spring Tower.
Carilion’s new facility will have 11 specialized Philips interventional suites, allowing physicians to treat patients with complex cardiovascular conditions closer to home while optimizing the clinical, operational and overall performance of the health system’s cardiovascular service line. This investment illustrates Carilion’s commitment to empowering its clinicians with innovative technology to provide quality care for its communities.
Carilion’s comprehensive hospital network, primary and specialty physician practices and other complementary services deliver high-quality, patient-centered care. The flagship hospital, Carilion Roanoke Memorial Hospital, totals 718 beds. The Carilion Clinic Cardiovascular Institute will relocate to this new facility and provide state-of-the art heart, lung and vascular services to the region. Services include inpatient nursing care, cardiovascular ORs, cardiac catheterization and electrophysiology labs.
Along with caring for the local community, Carilion Roanoke Memorial Hospital is the major referral center and only Level 1 Trauma Center for patients in the region who require specialized medical care. It also has the only hybrid operating rooms available in Southwest Virginia. Equipped with these new Philips solutions, the highly skilled medical staff will be able to continue handling complex cases or procedures that may not be available at surrounding healthcare facilities.
“At Carilion Clinic, we are committed to improving the health of the communities we serve. We know that collaboration is an essential component to realizing this vision,” said Marguerite Underwood, Vice President for the Carilion Clinic Cardiovascular Institute. “Working with Philips ensures that we will be able to set the standard in cardiovascular care, not only today, but well into the future.”
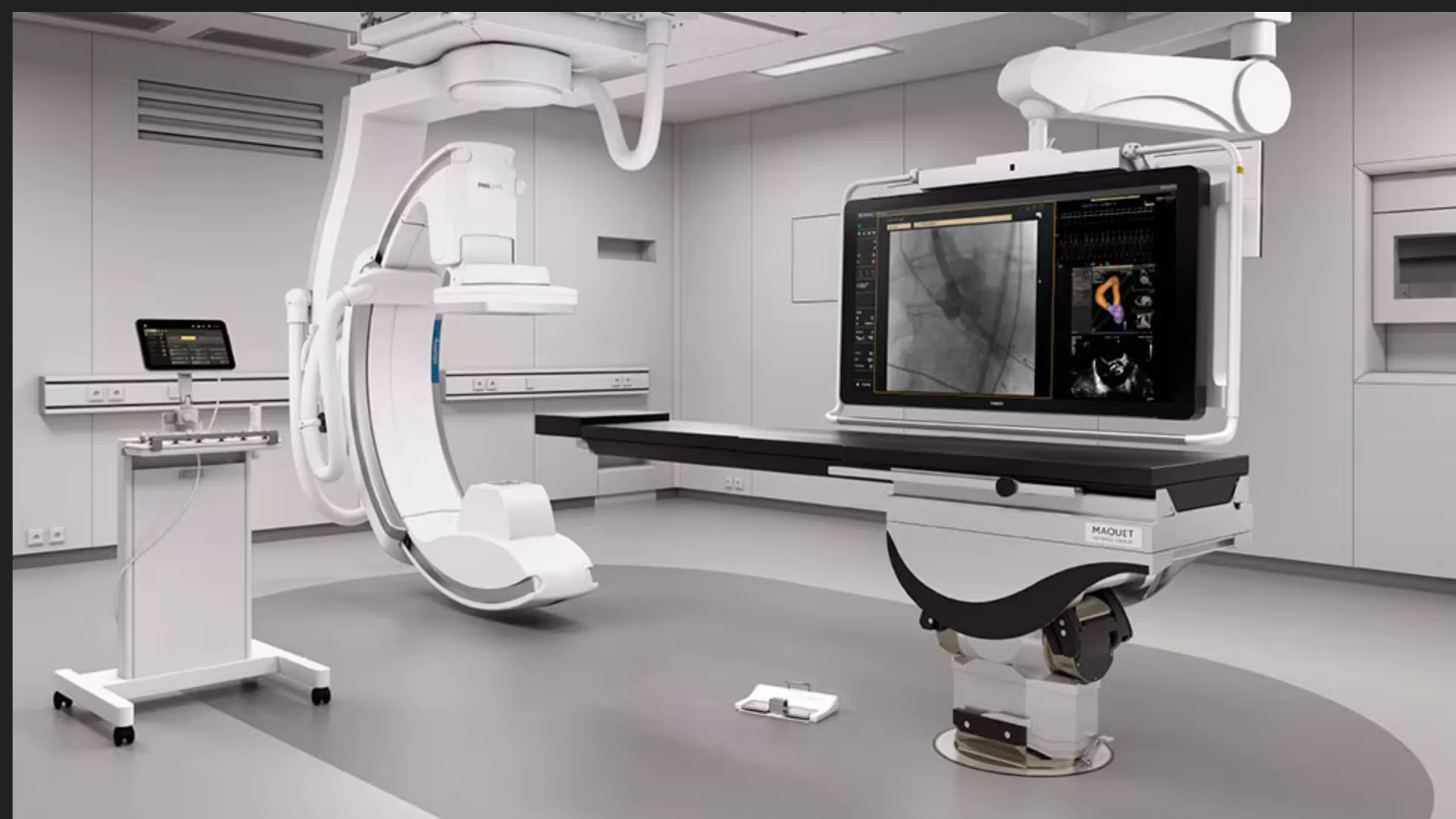
The new labs are the foundation of the integrated Philips ecosystem of solutions for cardiovascular care and include the Philips Azurion Image-Guided Therapy System, along with EPIQ CVx cardiology ultrasound system with ultrasound enabled AI capabilities, IntraSight with SyncVision for IVUS imaging and physiology and interventional tools. Together, the technology provides exceptional imaging with reduced X-ray dose [1], improved workflow and more efficient turnaround times [2], offering a high level of clinical confidence. A service program will reduce maintenance complexity, keeping systems up to date with the latest software. Furthermore, Carilion will be able to leverage ongoing clinical education for its staff.
“For many years, Carilion has been recognized for delivering exceptional cardiac care. Maintaining the highest standard of care means continuous growth and innovation, making healthcare delivery more efficient and effective,” said Jeff DiLullo, Chief Region Leader, Philips North America. “The Crystal Spring Tower expansion illustrates Carilion’s commitment to its staff and patients, giving clinicians access to world-class technology while providing a level of care not available anywhere else in the region.”