Centinel Spine®, LLC, (“the Company”) a leading global medical device company addressing cervical and lumbar spinal disease by providing the most robust and clinically-proven total disc replacement technology platform in the world (prodisc®), today announced the completion of the 1,000th procedure in the United States with the Company’s latest FDA-approved total disc replacement (TDR) system of prodisc® cervical solutions, prodisc C Vivo and prodisc C SK. The milestone comes two months after the Company completed 500 procedures with the system and validates the benefit of matching the implant to meet surgeon preference and patient needs. Since the limited launch of the new prodisc C Vivo and prodisc C SK system was initiated in September 2022, procedure volume with the new system has continued to grow exponentially—with an increase of over 60% in 1Q 2023 vs 4Q 2022.
According to spine surgeon Bradley Duhon, M.D., of Lone Tree, Colorado, “prodisc C Vivo comes with the trusted reputation of its Centinel Spine predecessors, but now with much easier implantation. It has become my ‘go-to’ disc replacement because it comes with very little bone work or prep. This not only makes for easier implantation, but also less endplate disruption, less bleeding, and less potential for heterotopic ossification.”
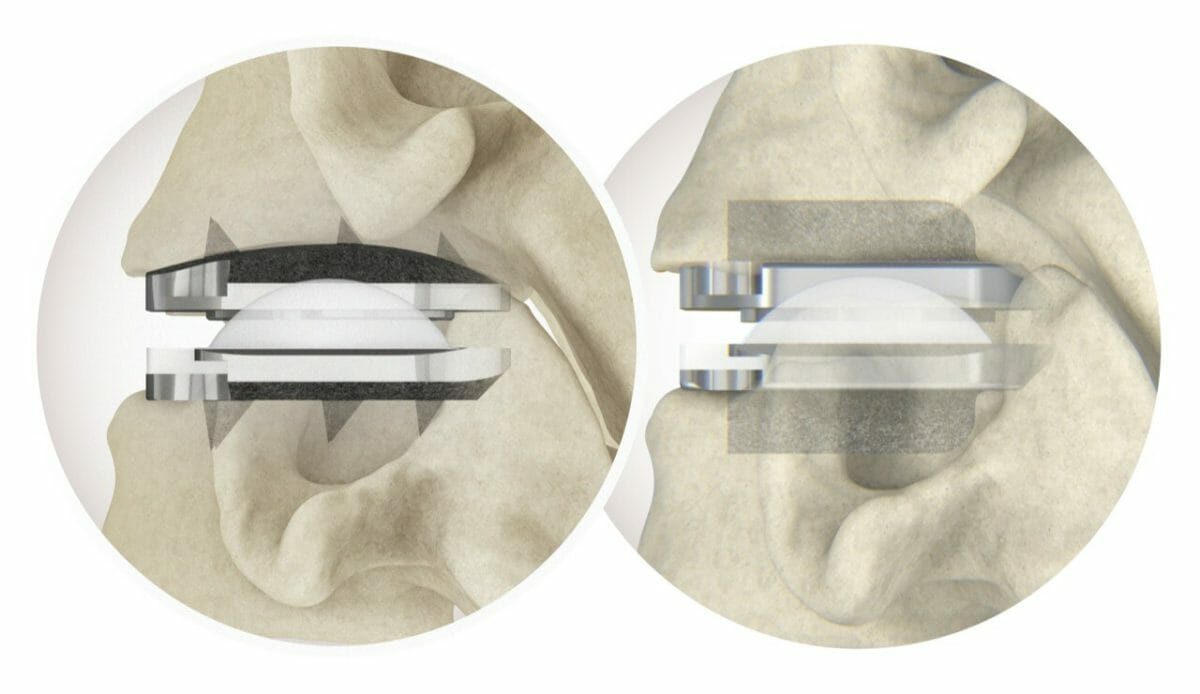
The prodisc C Vivo system has been in clinical use internationally since 2009 and is currently one of the most frequently implanted TDR devices in the world. The device has keel-less fixation and combines a unique anatomically-designed superior endplate with lateral spikes to optimize fit and provide immediate fixation.
The prodisc C SK device features a flat endplate design for optimized implant positioning that allows surgeons to address individual patient anatomy—and a low-profile central keel that provides immediate fixation and enables a streamlined keel preparation technique.
“The Match-the-Disc™ system of prodisc C Vivo and prodisc C SK, along with the original prodisc C, is exhibiting exponential growth,” observes Centinel Spine CEO Steve Murray. “With the addition of more instrument sets throughout the year, we will strive to support the unprecedented demand for this unique, paradigm-shifting total disc replacement system.”
The prodisc portfolio is the most extensive TDR system in the world, offering both cervical and lumbar implants with anatomically-differentiated designs. Centinel Spine is the only company with FDA approval for both cervical and lumbar TDR systems. All of the prodisc cervical and lumbar devices incorporate prodisc CORE technology, the basis behind the predictable clinical outcomes of the prodisc platform after 30 years and over 225,000 implantations worldwide.*