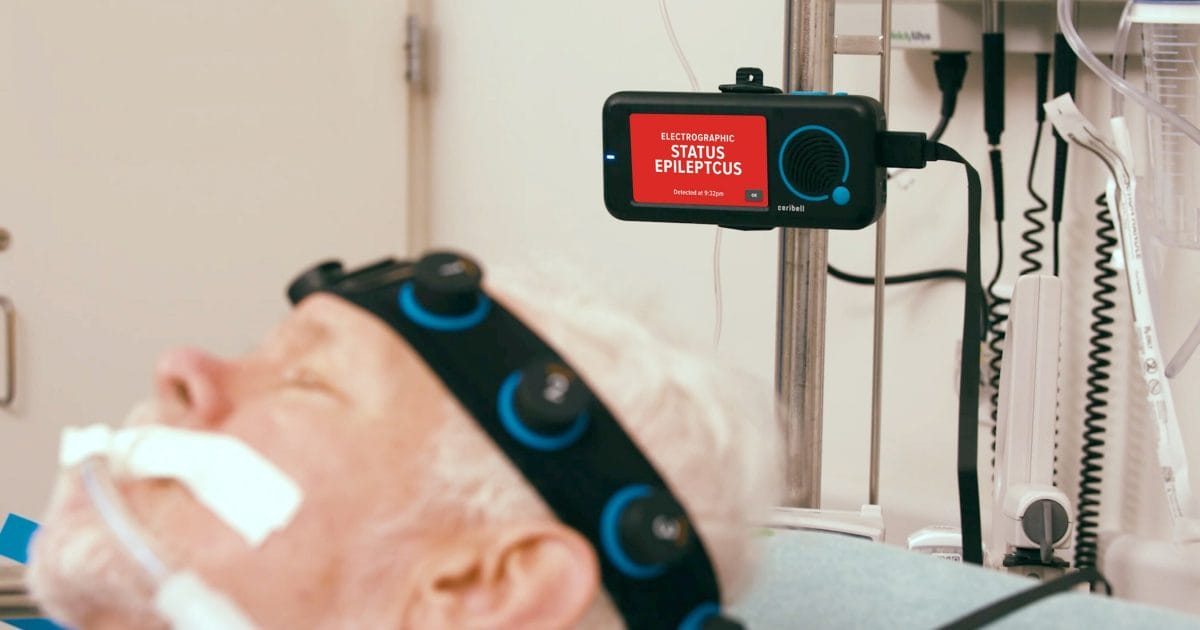
Ceribell, Inc.® today announced the commercial release of its new AI algorithm, ClarityPro™, accompanying the availability of potential additional reimbursement for eligible Medicare patients.
ClarityPro is an AI-based software algorithm within the Ceribell EEG system that analyzes EEG waveforms to diagnose electrographic status epilepticus (ESE). It is the first acute care device to be FDA indicated for stand-alone diagnosis. It is also the first critical care monitoring device to secure both FDA Breakthrough Designation and Centers for Medicare & Medicaid Services (CMS) New Technology Add-on Payment (NTAP) reimbursement, based on the substantial clinical benefit over existing devices for suspected seizure patients. With a unique code, hospitals may now be reimbursed up to $913.90 per eligible Medicare patient when using this groundbreaking technology.
ClarityPro can continuously monitor patients’ brain activity for suspected seizures and can diagnose and alert for ESE, giving clinicians at the bedside and remotely the vital, real-time data needed to rapidly identify and treat seizures and to evaluate the efficacy of seizure medication. With automated cues highlighting significant areas of concern within the EEG, ClarityPro provides insights to aid neurologists during EEG review, facilitating a quick response when escalation of care is needed. Importantly, the Ceribell system also enables hospitals with ClarityPro to more easily expand brain monitoring capabilities to acute care departments at a fraction of the cost of conventional EEG, while also helping to reduce use of unnecessary medication, intubations and patient transfers, and shortening ICU length of stay.1-4
“Every minute counts when it comes to treating status epilepticus. Ceribell is dedicated to expanding access to neurodiagnostics, which is essential to improving patient care and outcomes,” said Jane Chao, Ph.D.,Ceribell’s Co-founder and CEO. “ClarityPro harnesses the power of AI to bring acute seizure management to a new level, helping clinicians make precise treatment decisions while also helping hospitals extend care to more patients.”
For additional information about ClarityPro, the FDA Breakthrough Device program, the CMS NTAP program, and status epilepticus, visit https://ceribell.com/ClarityPro.