February 1, 2021
Editors Note: Why Are Medical Device Multinationals Choking Disruptive Technology and Killing Innovation? Challenges to Innovation in Medical Device Technology Published in the Journal of Endovascular Therapy. View Here.
By Prof Sherif Sultan, MB BCh MCh MD FRCSI DEVS FISVS FASA DMD FRCS/Vasc EBQS/Vasc FAARM FACS FEVBS PhD
Professor of Vascular & Endovascular Surgery
National University of Ireland
Chairman of Western Vascular Institute
University Hospital Galway, NUIG
The Galway Clinic, RCSI
The three individual innate traits that mandate our future goals and act as our natural differentiators are expertise, creative thinking, and intrinsic motivation (Figure 1). Innovators tend to have outstanding educational credentials and often a state of the art facility, but there is no hope for accomplishment without inspiration. Therefore, we believe that “excellence is not competitive.”
Our autonomy and freedom foster creativity and heightens our intrinsic motivation and sense of ownership. Intellectual Space allows us to approach problems through our expertise and creative thinking. The intrinsic motivation of relishing a challenge, and our drive to crack a challenging situation that no one else has been able to solve is self-propelling. The currency of Innovation is not in denominated dollars or euros. Instead, Innovation is driven by the individual, internal motivation to create something that heretofore did not exist. It is this sort of innate stimulus that Mark Twain referred to when he said: “Find a job you enjoy doing, and you will never have to work a day in your life.”
Vascular interventionalists face daily challenges while managing complex vascular procedures. Overcoming these challenges requires business acumen and, imaginative thinking, which only occur with expertise and motivation. However, legal regulations and systemic processes often hinder the path forward, including narrow-minded views from multinationals corporations. Creative thinking is a natural talent, where we try out solutions that depart from the status quo and turn problems into challenges, often combining knowledge from different fields. An aptitude for technical problem-solving requires proficiency in medicine, engineering, chemistry, biology, and biochemistry, which was attained through formal education, practical experience, and continuous interaction with other professionals. The more extensive the network of scientific exploration, that we are involved in, the better our expertise and knowledge.
Existing Medtech companies are directed by senior managers, who focus mostly on growth and profit. Therefore, they are unwilling to invest in the long game and aim to acquire technologies at a more mature development stage to gain quick results. They focus on catering to the needs of the ‘early and late majority,’ which is where the most money is made. The problem is that both ‘the early and the late majority’ are conservative, and they tend to avoid disruptive change. The majority of physicians are willing to improve current technology; however, their skepticism overrides their adoption of innovative techniques.
Furthermore, physicians are becoming increasingly aware of cost-effectiveness. This strategy discourages Innovation, as Innovation might not be viable economically at its early phase. This new norm chokes disruption and kills Innovation, and breeds a culture in which physicians are increasingly reserved about new ideas, met with criticism, pessimistic attitudes, and cynical skepticism. Physician adaptation of Innovation is discouraged at an organizational level, and although it may be given lip service, attempts to introduced technologies are stifled by red tape and the hurdles that physicians must jump through.
Newmarket leaders have been transforming their tactics from focusing on the return of investment; to return on failure. This 360-degree vision allows reverse engineering of challenges and optimizing of losses, in an attempt to save millions. New ideas are not always met with open minds. They can be subjected to continuous harsh criticism and an excruciating critique from teams of highly paid lawyers and regulators. It is an exciting dynamic when medical device multinationals hold sizeable intellectual property portfolios. Unfortunately, this sort of negative bias impacts on creativity and undermines intrinsic motivation.
Infighting, politics, and gossip are damaging to creativity. Political problems abound between multinationals and innovators and leave us feeling that medical device moguls agendas threaten progress. Politics are always in the way of open communication, obstructing information flow from point A to point B. This culminates in knowledge inertia, and proficiency suffers. Therefore, organizational cultures must promote creative thinking, embrace uncertainty and encourage exploration with a safe environment for passionate innovators to fail.
Regrettably, multinational medical device conglomerates stifle the very Innovation they seek through deep-rooted habits impeding creativity and avoiding risk-taking.
However, medical device connectivity is a significant challenge for innovations. Cyber threats are a constant concern for any health organization using connected systems. Vigilance and preparedness are essential to counter the risk. The benefits of connectivity are immeasurable, permitting patients to achieve more personalized care. Software architects for medical devices are caught between two extremes.
On the one hand, there are endless benefits from connectivity and big data, that unlock exciting opportunities. However, cyber-risks raise unprecedented barriers and leave companies vulnerable. The only solution is cross-industry collaboration.
Smart medical innovators should scrutinize the majority’s needs and start with small, incremental improvements to convince others to upgrade. Insidious changes will eventually lead to a robust and safer environment for Innovation.
The maintenance of quality for Innovation in a healthcare setting is challenging but necessary. Process management systems, such as ISO 9000, Six Sigma, and Lean, are shown to increase reliability. However, without flexibility, they have the potential to suppress radical Innovation. Systems that are good for standardization are not always suitable for Innovation, and we must create a mix of both for further exploration and implementation.
Young innovators should avoid unnecessary or unsolicited propagation of their ideas to prevent the nay-sayers who could crush their ideas rather than nurture them. The corporate world is a competitive playing-field where power struggles and egos value criticism above praise, and executives use negativity towards others to further their ambitions. Introducing a freshly hatched idea into that scenario could be detrimental to confidence and motivation. Remember that corporate managers are not trained to be innovation leaders, and they tend to look for flaws in ideas rather than teasing out any potential.
We advise the greater vascular community and relevant authorities to promote new ideas and use available resources to create a space where an idea can grow and flourish to serve our patients’ more significant needs.
“To innovate is sublime and to see our patients recover better and suffer less is the greatest joy of our profession.” Juan Carlos Parodi, July 2019
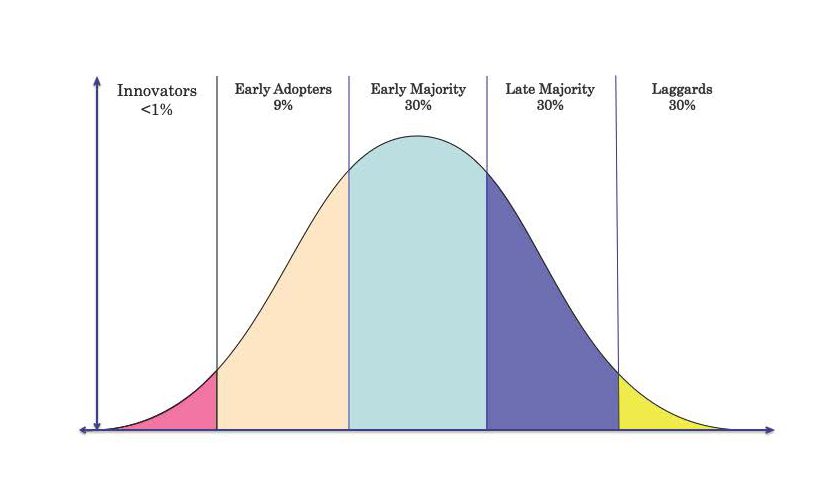
Figure 1: Diffusion of innovation and product adoption cycle in greater vascular community.
Innovators and early adaptors are Interventional Cardiologists, Endovascular Key opinion leaders, Neuroradiologists. The Early Majority are Vascular and Endovascular specialists, Late