Circle Cardiovascular Imaging Inc. and DiA Imaging Analysis Ltd. have announced a multi-year partnership that leverages the companies’ synergies in cardiac AI and data analytics.
As a global leader in cardiovascular imaging reading and reporting for Cardiac MR and Cardiac CT, the company has now expanded its rich product offering to a complete cardiovascular imaging portfolio, with the addition of DiA’s FDA-cleared and CE-marked LVivo™ Toolbox – a line of innovative AI-based cardiac ultrasound solutions. This collaboration will deliver expanded state-of-the-art multi-modality imaging solutions to Circle Cardiovascular Imaging’s customers, while providing new opportunities for physicians, patients, and hospitals worldwide.
“By adding DiA’s solutions, Circle Cardiovascular Imaging is broadening its extensive portfolio of cardiovascular MR and CT, to include ultrasound imaging functionality, offerings that are unparalleled in the market,” said Greg Ogrodnick, CEO of Circle Cardiovascular Imaging.
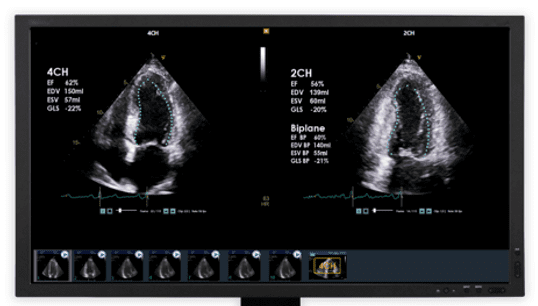
The company will offer its customers DiA’s LVivo Toolbox, as set of cardiac ultrasound AI solutions which will include LVivo Seamless™ – a unique system that runs “behind the scenes,” automatically selecting the optimal cardiac ultrasound views and generating automated quantifications and indications of both left and right ventricles. The system then immediately extracts these results to the echo reports.
The solutions of both companies are vendor-neutral, running on any scanner, and they can easily integrate into hospital and enterprise sites, with deployments that work with any IT infrastructure.
“Joining forces with Circle Cardiovascular Imaging will accelerate our mission to rapidly deploy health providers with our most advanced and most accurate cardiac ultrasound AI solutions, which will simplify workflows and improve patient outcomes,” said Hila Goldman-Aslan, CEO of DiA Imaging Analysis.
DiA will spotlight its AI-enabled solutions across its imaging portfolio at the upcoming American Society of Echocardiography (ASE) 2021 virtual event (June 18-21, 2021).