October 16, 2020
CLAAS Stroke Prevention Technology: Conformal Medical, Inc., a privately-held medical device company developing next-generation left atrial appendage closure (LAAC) technology, announced positive clinical data from First-in-Human studies performed in the U.S. and Prague. Data were presented at the 32nd Transcatheter Cardiovascular Therapeutics (TCT) meeting in two abstracts by William Gray, MD (Lankenau Heart Institute, Wynnewood, PA) and Vivek Reddy MD (Mount Sinai Hospital, NY). Click here to view the 2020 TCT abstract presentations.
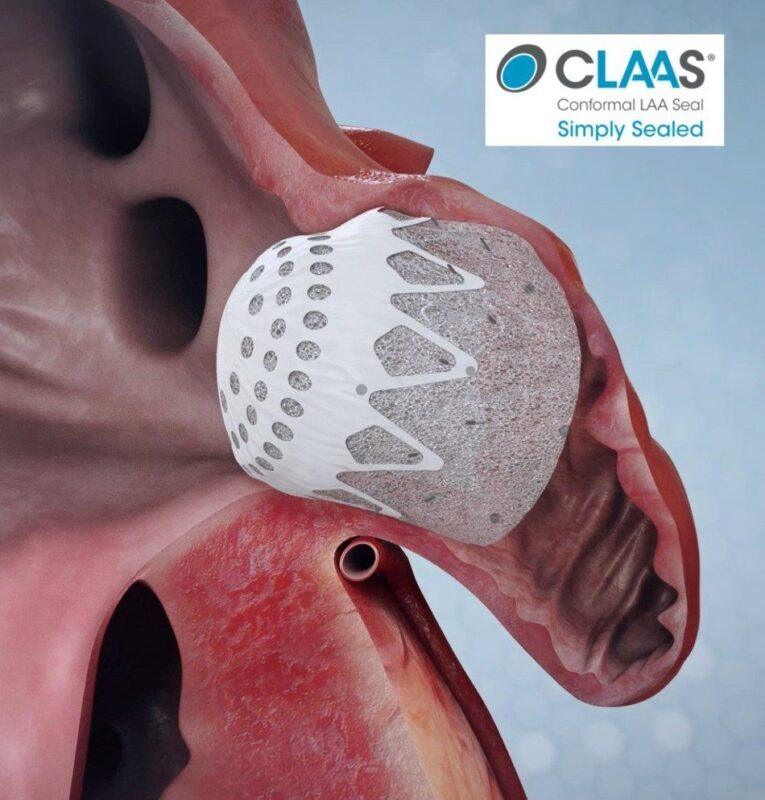
Both studies investigated Conformal’s CLAAS Technology, a next-generation solution for sealing the left atrial appendage (LAA) in patients with atrial fibrillation. Featuring a proprietary foam-based architecture, the CLAAS implant is designed to address the wide spectrum of LAA anatomies with only two sizes and provide simplified delivery without the need for procedural transesophageal echo (TEE), allowing physicians to perform LAAC without general anesthesia.
The Prague single-center study (n=15) investigated ICE-guided LAA closure under conscious sedation. The two sizes of the CLAAS device were successfully implanted in all patients, effectively addressing LAA diameters from 11mm to 28mm, without the need for procedural TEE. Additionally, all implants resulted in successful seals without device-related procedural complications.
“The foam-based CLAAS device allowed me to consistently seal each patient’s unique LAA anatomy,” commented Dr. Reddy. “Our study was performed using Intracardiac Echo (ICE) and fluoroscopy, without intraprocedural TEE or general anesthesia, and shows the potential of this device, which was easy to deliver and required just two sizes.”
The U.S. multi-center study enrolled 22 patients at four clinical sites. There were no device-related procedural complications, demonstrating closure of LAA diameters from 9mm to 31mm. In this study, procedural TEE imaging confirmed conformability with encouraging sealing results.
“LAA closure is becoming an important alternative to anticoagulation for patients with atrial fibrillation. Though first-generation devices have shown efficacy, they may have significant limitations in some patients,” stated Dr. Gray. “I am very pleased with the initial CLAAS experience. It has the potential for us to treat a broader spectrum of patients and to transform LAAC to a same day procedure.”
“We are pleased with the results shared today and look forward to initiating our pivotal trial to further validate the significant impact our innovation will bring to the LAA closure market,” stated Andy Levine, co-founder and chief executive officer of Conformal Medical.
In 2021, Conformal Medical will begin its randomized pivotal trial that will enroll approximately 1,400 patients at multiple sites globally to investigate the safety and effectiveness of the CLAAS technology compared to Boston Scientific’s Watchman products.