February 2, 2021
ClarifEye Augmented Reality Surgical Navigation, an industry-first solution to advance minimally-invasive spine procedures in the Hybrid Operating Room, such as Philips’ Hybrid Suite.
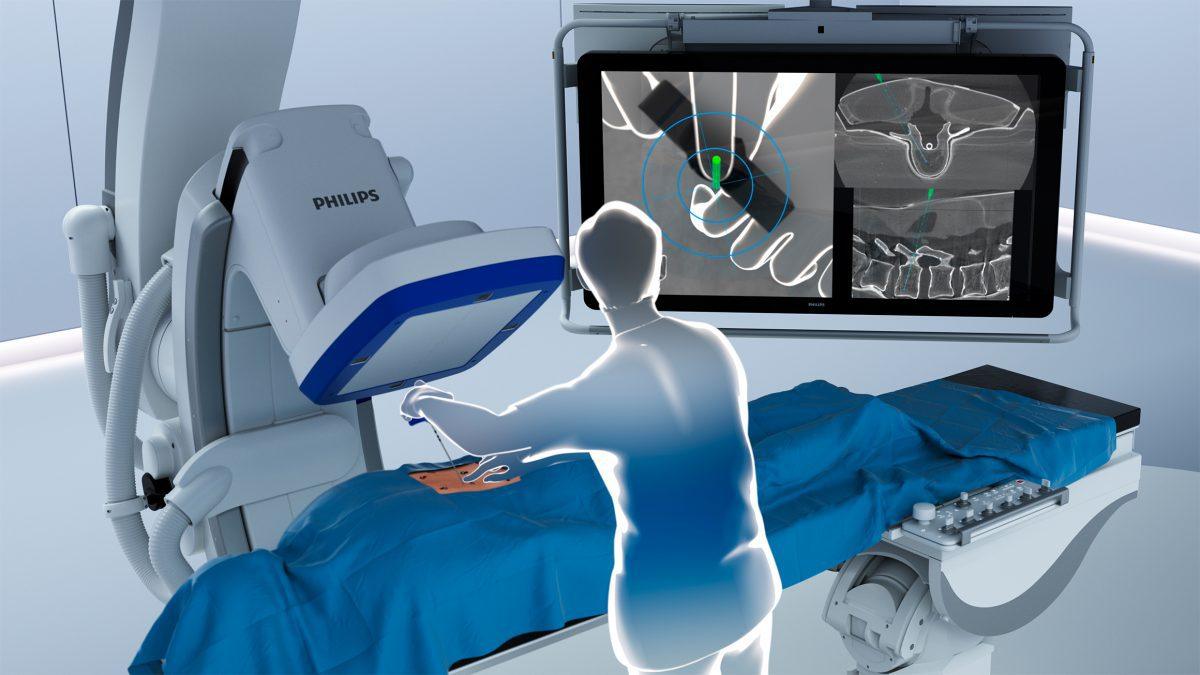
By combining superb 2D and 3D visualizations at low X-ray dose [3] with 3D augmented reality (AR), the unique solution provides live intra-operative visual feedback to support accurate placement of pedicle screws during spinal fusion procedures. During such procedures, two or more vertebrae in the spine are permanently connected to help improve stability, correct a deformity, or reduce pain. The solution is fully integrated into the Philips Azurion image-guided therapy platform, supporting efficient workflow with intra-procedural navigation and verification for accurate screw placement and reducing the need for post-operative CT scans.
Spine conditions can have a significant impact on quality of life and well-being, with severe cases leaving patients unable to walk or even move from their beds. Treatment is typically complex and delicate, with surgeons required to take particular care to avoid fragile neurological and vascular structures that are close to the spine.
By taking a minimally-invasive approach to spine surgery, patients can benefit from reduced postoperative pain, shortened hospital stays, reduced blood loss, and minimized soft tissue damage and scar tissue [4]. In addition, the intra-operative image guidance provided by solutions such as ClarifEye increases clinical accuracy, with patients subject to fewer revision surgeries compared to the current standard of care [1,2].
“In spine surgery, when you change your approach to a minimally invasive one, you also have to change the way you operate because you need another way to see inside the spine,” said Dr. Pietro Scarone, neurosurgeon at Ente Ospedaliero Cantonale in Lugano, Switzerland. “With ClarifEye, the technology adapts to the needs of the surgeon, rather than the surgeon adapting to the requirements of the technology.”
“Augmented reality surgical navigation helps us to place pedicle screws in positions where we actually couldn’t or wouldn’t do otherwise,” said Dr. Adrian Elmi-Terander, neurosurgeon in the department of Neurosurgery at the Karolinska University Hospital in Stockholm, Sweden.
Four high-resolution optical cameras are used to augment the surgical field with 3D cone-beam CT imaging, without the need for additional X-ray. The system combines the view of the surgical field with the internal 3D view of the patient to construct a 3D augmented-reality view of the patient’s external and internal anatomy. Consistent tracking of the patient is ensured by video tracking of non-invasive markers placed on the skin. The system then visualizes the tip of the ClarifEye Needle as it is navigated along the planned path in the spine.
“Post-operative CT scans to check implant placements are no longer necessary,” said Prof. Dr. Andreas Seekamp, Director of the Orthopaedic and Emergency Surgery clinic at the University Medical Center Schleswig-Holstein in Kiel, Germany. “As soon as surgery has been performed, we can be 100% sure that the implants are in place, thanks to the high quality of the intra-operative cone beam CT image and positioning flexibility of the system.”
“Through co-creation with our clinical partners we’ve developed an innovative integrated solution that has the potential to improve outcomes and reduce costs for minimally invasive spine procedures,” said Ronald Tabaksblat, General Manager Image Guided Therapy Systems at Philips. “With ClarifEye we are delivering on our strategy of expanding minimally invasive surgery into new clinical areas. The solution is built on the Philips next-generation Azurion image-guided therapy platform, enabling an unmatched level of integration and an intuitive experience for clinicians.”
To learn more from physicians using advanced imaging and navigation solutions in spine surgeries, register here for the educational webinar taking place on Thursday, March 11th at 3:00pm CET.
For more information on Philips’ approach to bringing AR to minimally-invasive spine surgery, read this Q&A with Ronald Tabaksblat.
ClarifEye Augmented Reality Surgical Navigation is CE marked and 510(k) pending. This material is not for distribution in the U.S.A.
[1] Dea N, Fisher CG, Batke J, Strelzow J, Mendelsohn D, Paquette SJ, Kwon BK, Boyd MD, Dvorak MFS, Street JT. Economic evaluation comparing intraoperative cone beam C T based navigation and conventional fluoroscopy for the placement of spinal pedicle screws: a patient level data cost effectiveness anal ysis. The Spine Journal (2016) 16: 23 31.
[2] Fichtner J, Hofmann N, Rienmüller A, Buchmann N, Gempt J, Kirschke JS, Ringel F, Meyer B, Ryang Y M. Revision Rate of Misplaced Pedicle Screws of the Thoracolumbar SpineeComparison of Three Dimensional Fluoroscopy Navigation with Freehand Placement: A Systematic Analysis and Review of the Literature. World Neurosurg . (2018) 109: e24 e32.
[3] Nachabe R, Strauss K, Schueler B, Bydon M. Radiation dose and image quality comparison during spine surgery with two different, intraoperative 3D imaging navigation systems, J Appl Clin Med Phys 2019 Feb; 20(2): 136-145.
[4] Phan K, Rao PJ, Mobbs RJ. Percutaneous versus open pedicle screw fixation for treatment of thoracolumbar fractures: Systematic review and meta-analysis of comparative studies. Clinical neurology and neurosurgery. 2015. 135:85-92.